Iron complexes on:
[Wikipedia]
[Google]
[Amazon]
Iron () is a
 At least four allotropes of iron (differing atom arrangements in the solid) are known, conventionally denoted α, γ, δ, and ε.
At least four allotropes of iron (differing atom arrangements in the solid) are known, conventionally denoted α, γ, δ, and ε.
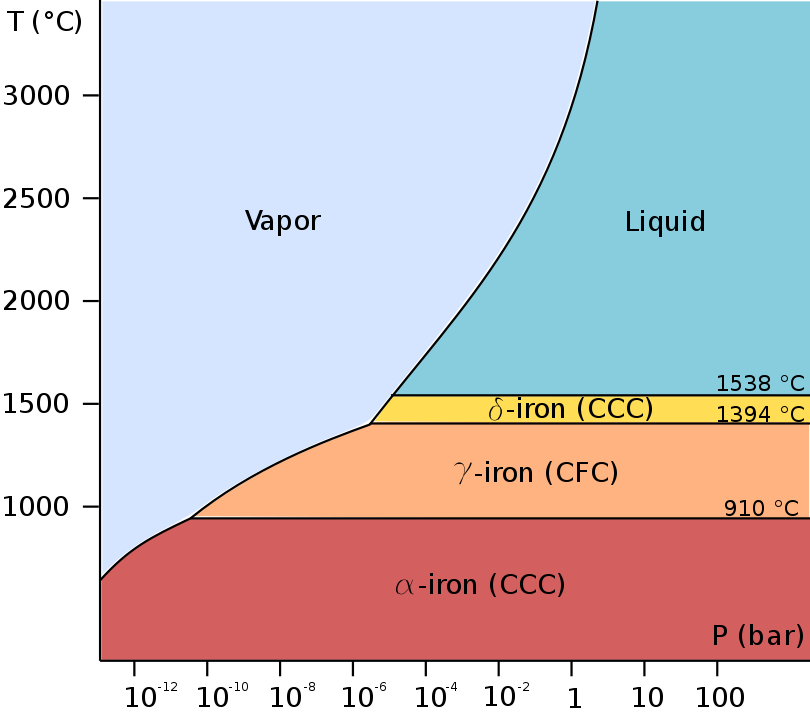 The first three forms are observed at ordinary pressures. As molten iron cools past its freezing point of 1538 °C, it crystallizes into its δ allotrope, which has a
The first three forms are observed at ordinary pressures. As molten iron cools past its freezing point of 1538 °C, it crystallizes into its δ allotrope, which has a
 Below its
Below its
 Metallic or
Metallic or
Mindat.org
 While iron is the most abundant element on Earth, most of this iron is concentrated in the inner and
While iron is the most abundant element on Earth, most of this iron is concentrated in the inner and
chemical element
A chemical element is a species of atoms that have a given number of protons in their nuclei, including the pure substance consisting only of that species. Unlike chemical compounds, chemical elements cannot be broken down into simpler sub ...
with symbol
A symbol is a mark, sign, or word that indicates, signifies, or is understood as representing an idea, object, or relationship. Symbols allow people to go beyond what is known or seen by creating linkages between otherwise very different conc ...
Fe (from la, ferrum) and atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of every ...
26. It is a metal
A metal (from Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typi ...
that belongs to the first transition series and group 8 of the periodic table
The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of ...
. It is, by mass, the most common element on Earth
Earth is the third planet from the Sun and the only astronomical object known to harbor life. While large volumes of water can be found throughout the Solar System, only Earth sustains liquid surface water. About 71% of Earth's surf ...
, right in front of oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as we ...
(32.1% and 30.1%, respectively), forming much of Earth's outer and inner core
Earth's inner core is the innermost geologic layer of planet Earth. It is primarily a solid ball with a radius of about , which is about 20% of Earth's radius or 70% of the Moon's radius.
There are no samples of Earth's core accessible for ...
. It is the fourth most common element in the Earth's crust.
In its metallic state, iron is rare in the Earth's crust
Earth's crust is Earth's thin outer shell of rock, referring to less than 1% of Earth's radius and volume. It is the top component of the lithosphere, a division of Earth's layers that includes the crust and the upper part of the mantle. The ...
, limited mainly to deposition by meteorites
A meteorite is a solid piece of debris from an object, such as a comet, asteroid, or meteoroid, that originates in outer space and survives its passage through the atmosphere to reach the surface of a planet or moon. When the original object e ...
. Iron ore
Iron ores are rocks and minerals from which metallic iron can be economically extracted. The ores are usually rich in iron oxides and vary in color from dark grey, bright yellow, or deep purple to rusty red. The iron is usually found in the ...
s, by contrast, are among the most abundant in the Earth's crust, although extracting usable metal from them requires kiln
A kiln is a thermally insulated chamber, a type of oven, that produces temperatures sufficient to complete some process, such as hardening, drying, or chemical changes. Kilns have been used for millennia to turn objects made from clay int ...
s or furnaces capable of reaching or higher, about higher than that required to smelt copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish ...
. Humans started to master that process in Eurasia
Eurasia (, ) is the largest continental area on Earth, comprising all of Europe and Asia. Primarily in the Northern and Eastern Hemispheres, it spans from the British Isles and the Iberian Peninsula in the west to the Japanese archipelag ...
during the 2nd millennium BCE
The 2nd millennium BC spanned the years 2000 BC to 1001 BC.
In the Ancient Near East, it marks the transition from the Middle to the Late Bronze Age.
The Ancient Near Eastern cultures are well within the historical era:
The first half of the mil ...
and the use of iron tool
A tool is an object that can extend an individual's ability to modify features of the surrounding environment or help them accomplish a particular task. Although many animals use simple tools, only human beings, whose use of stone tools dates ba ...
s and weapon
A weapon, arm or armament is any implement or device that can be used to deter, threaten, inflict physical damage, harm, or kill. Weapons are used to increase the efficacy and efficiency of activities such as hunting, crime, law enforcement, ...
s began to displace copper alloys, in some regions, only around 1200 BCE. That event is considered the transition from the Bronze Age
The Bronze Age is a historic period, lasting approximately from 3300 BC to 1200 BC, characterized by the use of bronze, the presence of writing in some areas, and other early features of urban civilization. The Bronze Age is the second pri ...
to the Iron Age
The Iron Age is the final epoch of the three-age division of the prehistory and protohistory of humanity. It was preceded by the Stone Age (Paleolithic, Mesolithic, Neolithic) and the Bronze Age (Chalcolithic). The concept has been mostly appl ...
. In the modern world, iron alloys, such as steel, stainless steel, cast iron
Cast iron is a class of iron– carbon alloys with a carbon content more than 2%. Its usefulness derives from its relatively low melting temperature. The alloy constituents affect its color when fractured: white cast iron has carbide impu ...
and special steels, are by far the most common industrial metals, because of their mechanical properties and low cost. The iron and steel industry is thus very important economically, and iron is the cheapest metal, with a price of a few dollars per kilogram or per pound (see Metal#uses).
Pristine and smooth pure iron surfaces are mirror-like silvery-gray. However, iron reacts readily with oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as we ...
and water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as ...
to give brown to black hydrated iron oxide
Iron oxides are chemical compounds composed of iron and oxygen. Several iron oxides are recognized. All are black magnetic solids. Often they are non-stoichiometric. Oxyhydroxides are a related class of compounds, perhaps the best known of w ...
s, commonly known as rust
Rust is an iron oxide, a usually reddish-brown oxide formed by the reaction of iron and oxygen in the catalytic presence of water or air moisture. Rust consists of hydrous iron(III) oxides (Fe2O3·nH2O) and iron(III) oxide-hydroxide (FeO(OH), ...
. Unlike the oxides of some other metals that form passivating layers, rust occupies more volume than the metal and thus flakes off, exposing more fresh surfaces for corrosion. Although iron readily reacts, high purity iron, called electrolytic iron, has better corrosion resistance.
The body of an adult human contains about 4 grams (0.005% body weight) of iron, mostly in hemoglobin
Hemoglobin (haemoglobin BrE) (from the Greek word αἷμα, ''haîma'' 'blood' + Latin ''globus'' 'ball, sphere' + ''-in'') (), abbreviated Hb or Hgb, is the iron-containing oxygen-transport metalloprotein present in red blood cells (erythrocyte ...
and myoglobin
Myoglobin (symbol Mb or MB) is an iron- and oxygen-binding protein found in the cardiac and skeletal muscle tissue of vertebrates in general and in almost all mammals. Myoglobin is distantly related to hemoglobin. Compared to hemoglobin, myoglobi ...
. These two protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, respon ...
s play essential roles in vertebrate
Vertebrates () comprise all animal taxon, taxa within the subphylum Vertebrata () (chordates with vertebral column, backbones), including all mammals, birds, reptiles, amphibians, and fish. Vertebrates represent the overwhelming majority of the ...
metabolism
Metabolism (, from el, μεταβολή ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run c ...
, respectively oxygen transport
Blood is a body fluid in the circulatory system of humans and other vertebrates that delivers necessary substances such as nutrients and oxygen to the cells, and transports metabolic waste products away from those same cells. Blood in the ci ...
by blood
Blood is a body fluid in the circulatory system of humans and other vertebrates that delivers necessary substances such as nutrients and oxygen to the cells, and transports metabolic waste products away from those same cells. Blood in th ...
and oxygen storage in muscle
Skeletal muscles (commonly referred to as muscles) are Organ (biology), organs of the vertebrate muscular system and typically are attached by tendons to bones of a skeleton. The muscle cells of skeletal muscles are much longer than in the other ...
s. To maintain the necessary levels, human iron metabolism requires a minimum of iron in the diet. Iron is also the metal at the active site of many important redox
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate (chemistry), substrate change. Oxidation is the loss of Electron, electrons or an increase in the oxidation state, while reduction ...
enzymes dealing with cellular respiration
Cellular respiration is the process by which biological fuels are oxidised in the presence of an inorganic electron acceptor such as oxygen to produce large amounts of energy, to drive the bulk production of ATP. Cellular respiration may be des ...
and oxidation and reduction in plants and animals.
Chemically, the most common oxidation states of iron are iron(II) and iron(III). Iron shares many properties of other transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that c ...
s, including the other group 8 elements, ruthenium
Ruthenium is a chemical element with the symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most other chemic ...
and osmium
Osmium (from Greek grc, ὀσμή, osme, smell, label=none) is a chemical element with the symbol Os and atomic number 76. It is a hard, brittle, bluish-white transition metal in the platinum group that is found as a trace element in alloys, mos ...
. Iron forms compounds in a wide range of oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. ...
s, −2 to +7. Iron also forms many coordination compound
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many ...
s; some of them, such as ferrocene
Ferrocene is an organometallic compound with the formula . The molecule is a complex consisting of two cyclopentadienyl rings bound to a central iron atom. It is an orange solid with a camphor-like odor, that sublimes above room temperature, ...
, ferrioxalate
Ferrioxalate or trisoxalatoferrate(III) is a trivalent anion with formula . It is a transition metal complex consisting of an iron atom in the +3 oxidation state and three bidentate oxalate ions anions acting as ligands.
The ferrioxalate ani ...
, and Prussian blue
Prussian blue (also known as Berlin blue, Brandenburg blue or, in painting, Parisian or Paris blue) is a dark blue pigment produced by oxidation of ferrous ferrocyanide salts. It has the chemical formula Fe Cyanide.html" ;"title="e(Cyanide">CN ...
, have substantial industrial, medical, or research applications.
Characteristics
Allotropes
 The first three forms are observed at ordinary pressures. As molten iron cools past its freezing point of 1538 °C, it crystallizes into its δ allotrope, which has a
The first three forms are observed at ordinary pressures. As molten iron cools past its freezing point of 1538 °C, it crystallizes into its δ allotrope, which has a body-centered cubic
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
There are three main varieties of ...
(bcc) crystal structure
In crystallography, crystal structure is a description of the ordered arrangement of atoms, ions or molecules in a crystalline material. Ordered structures occur from the intrinsic nature of the constituent particles to form symmetric patterns t ...
. As it cools further to 1394 °C, it changes to its γ-iron allotrope, a face-centered cubic
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
There are three main varieties o ...
(fcc) crystal structure, or austenite
Austenite, also known as gamma-phase iron (γ-Fe), is a metallic, non-magnetic allotrope of iron or a solid solution of iron with an alloying element. In plain-carbon steel, austenite exists above the critical eutectoid temperature of 1000 ...
. At 912 °C and below, the crystal structure again becomes the bcc α-iron allotrope.
The physical properties of iron at very high pressures and temperatures have also been studied extensively, because of their relevance to theories about the cores of the Earth and other planets. Above approximately 10 GPa and temperatures of a few hundred kelvin or less, α-iron changes into another hexagonal close-packed
In geometry, close-packing of equal spheres is a dense arrangement of congruent spheres in an infinite, regular arrangement (or lattice). Carl Friedrich Gauss proved that the highest average density – that is, the greatest fraction of space occ ...
(hcp) structure, which is also known as ε-iron. The higher-temperature γ-phase also changes into ε-iron, but does so at higher pressure.
Some controversial experimental evidence exists for a stable β phase at pressures above 50 GPa and temperatures of at least 1500 K. It is supposed to have an orthorhombic
In crystallography, the orthorhombic crystal system is one of the 7 crystal systems. Orthorhombic lattices result from stretching a cubic lattice along two of its orthogonal pairs by two different factors, resulting in a rectangular prism with ...
or a double hcp structure. (Confusingly, the term "β-iron" is sometimes also used to refer to α-iron above its Curie point, when it changes from being ferromagnetic to paramagnetic, even though its crystal structure has not changed.)
The inner core
Earth's inner core is the innermost geologic layer of planet Earth. It is primarily a solid ball with a radius of about , which is about 20% of Earth's radius or 70% of the Moon's radius.
There are no samples of Earth's core accessible for ...
of the Earth
Earth is the third planet from the Sun and the only astronomical object known to harbor life. While large volumes of water can be found throughout the Solar System, only Earth sustains liquid surface water. About 71% of Earth's surf ...
is generally presumed to consist of an iron-nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow ...
alloy
An alloy is a mixture of chemical elements of which at least one is a metal. Unlike chemical compounds with metallic bases, an alloy will retain all the properties of a metal in the resulting material, such as electrical conductivity, ductilit ...
with ε (or β) structure.
Melting and boiling points
The melting and boiling points of iron, along with itsenthalpy of atomization
In chemistry, the enthalpy of atomization (also atomisation in British English) is the enthalpy change that accompanies the total separation of all atoms in a chemical substance (either an element or a compound). This is often represented by th ...
, are lower than those of the earlier 3d elements from scandium
Scandium is a chemical element with the symbol Sc and atomic number 21. It is a silvery-white metallic d-block element. Historically, it has been classified as a rare-earth element, together with yttrium and the Lanthanides. It was discovere ...
to chromium
Chromium is a chemical element with the symbol Cr and atomic number 24. It is the first element in group 6. It is a steely-grey, lustrous, hard, and brittle transition metal.
Chromium metal is valued for its high corrosion resistance and h ...
, showing the lessened contribution of the 3d electrons to metallic bonding as they are attracted more and more into the inert core by the nucleus;Greenwood and Earnshaw, p. 1116 however, they are higher than the values for the previous element manganese
Manganese is a chemical element with the symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese is a transition metal with a multifaceted array of industrial alloy u ...
because that element has a half-filled 3d sub-shell and consequently its d-electrons are not easily delocalized. This same trend appears for ruthenium but not osmium.
The melting point of iron is experimentally well defined for pressures less than 50 GPa. For greater pressures, published data (as of 2007) still varies by tens of gigapascals and over a thousand kelvin.
Magnetic properties
Curie point
In physics and materials science, the Curie temperature (''T''C), or Curie point, is the temperature above which certain materials lose their permanent magnetic properties, which can (in most cases) be replaced by induced magnetism. The Cur ...
of , α-iron changes from paramagnetic
Paramagnetism is a form of magnetism whereby some materials are weakly attracted by an externally applied magnetic field, and form internal, induced magnetic fields in the direction of the applied magnetic field. In contrast with this behavior, ...
to ferromagnetic
Ferromagnetism is a property of certain materials (such as iron) which results in a large observed magnetic permeability, and in many cases a large magnetic coercivity allowing the material to form a permanent magnet. Ferromagnetic materials ...
: the spins
The spins (as in having "the spins")Diane Marie Leiva. ''The Florida State University College of Education''Women's Voices on College Drinking: The First-Year College Experience"/ref> is an adverse reaction of intoxication that causes a state of ...
of the two unpaired electrons in each atom generally align with the spins of its neighbors, creating an overall magnetic field
A magnetic field is a vector field that describes the magnetic influence on moving electric charges, electric currents, and magnetic materials. A moving charge in a magnetic field experiences a force perpendicular to its own velocity and t ...
. This happens because the orbitals of those two electrons (d''z''2 and d''x''2 − ''y''2) do not point toward neighboring atoms in the lattice, and therefore are not involved in metallic bonding.
In the absence of an external source of magnetic field, the atoms get spontaneously partitioned into magnetic domain
A magnetic domain is a region within a magnetic material in which the magnetization is in a uniform direction. This means that the individual magnetic moments of the atoms are aligned with one another and they point in the same direction. When c ...
s, about 10 micrometers across, such that the atoms in each domain have parallel spins, but some domains have other orientations. Thus a macroscopic piece of iron will have a nearly zero overall magnetic field.
Application of an external magnetic field causes the domains that are magnetized in the same general direction to grow at the expense of adjacent ones that point in other directions, reinforcing the external field. This effect is exploited in devices that need to channel magnetic fields to fulfill design function, such as electrical transformer
A transformer is a passive component that transfers electrical energy from one electrical circuit to another circuit, or multiple circuits. A varying current in any coil of the transformer produces a varying magnetic flux in the transformer's ...
s, magnetic recording
Magnetic storage or magnetic recording is the storage of data on a magnetized medium. Magnetic storage uses different patterns of magnetisation in a magnetizable material to store data and is a form of non-volatile memory. The information is ac ...
heads, and electric motor
An electric motor is an electrical machine that converts electrical energy into mechanical energy. Most electric motors operate through the interaction between the motor's magnetic field and electric current in a wire winding to generate forc ...
s. Impurities, lattice defect
A crystallographic defect is an interruption of the regular patterns of arrangement of atoms or molecules in crystalline solids. The positions and orientations of particles, which are repeating at fixed distances determined by the unit cell para ...
s, or grain and particle boundaries can "pin" the domains in the new positions, so that the effect persists even after the external field is removed – thus turning the iron object into a (permanent) magnet
A magnet is a material or object that produces a magnetic field. This magnetic field is invisible but is responsible for the most notable property of a magnet: a force that pulls on other ferromagnetic materials, such as iron, steel, nic ...
.
Similar behavior is exhibited by some iron compounds, such as the ferrites Ferrite may refer to:
* Ferrite (iron), one of the allotropes of iron that is stable at room temperature and pressure, α-Fe
* Ferrite (magnet), a ferrimagnetic ceramic material
Ferrite family, a Spanish family that has members all over the world.
...
including the mineral magnetite
Magnetite is a mineral and one of the main iron ores, with the chemical formula Fe2+Fe3+2O4. It is one of the oxides of iron, and is ferrimagnetic; it is attracted to a magnet and can be magnetized to become a permanent magnet itself. With ...
, a crystalline form of the mixed iron(II,III) oxide (although the atomic-scale mechanism, ferrimagnetism
A ferrimagnetic material is a material that has populations of atoms with opposing magnetic moments, as in antiferromagnetism, but these moments are unequal in magnitude so a spontaneous magnetization remains. This can for example occur when ...
, is somewhat different). Pieces of magnetite with natural permanent magnetization (lodestone
Lodestones are naturally magnetized pieces of the mineral magnetite. They are naturally occurring magnets, which can attract iron. The property of magnetism was first discovered in antiquity through lodestones. Pieces of lodestone, suspen ...
s) provided the earliest compass
A compass is a device that shows the cardinal directions used for navigation and geographic orientation. It commonly consists of a magnetized needle or other element, such as a compass card or compass rose, which can pivot to align itself with ...
es for navigation. Particles of magnetite were extensively used in magnetic recording media such as core memories
Magnetic-core memory was the predominant form of random-access computer memory for 20 years between about 1955 and 1975.
Such memory is often just called core memory, or, informally, core.
Core memory uses toroids (rings) of a hard magnetic ...
, magnetic tape
Magnetic tape is a medium for magnetic storage made of a thin, magnetizable coating on a long, narrow strip of plastic film. It was developed in Germany in 1928, based on the earlier magnetic wire recording from Denmark. Devices that use mag ...
s, floppies
A floppy disk or floppy diskette (casually referred to as a floppy, or a diskette) is an obsolescent type of disk storage composed of a thin and flexible disk of a magnetic storage medium in a square or nearly square plastic enclosure lined wi ...
, and disks, until they were replaced by cobalt
Cobalt is a chemical element with the symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. The free element, ...
-based materials.
Isotopes
Iron has four stableisotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers ( mass number ...
s: 54Fe (5.845% of natural iron), 56Fe (91.754%), 57Fe (2.119%) and 58Fe (0.282%). 20-30 artificial isotopes have also been created. Of these stable isotopes, only 57Fe has a nuclear spin
In atomic physics, the spin quantum number is a quantum number (designated ) which describes the intrinsic angular momentum (or spin angular momentum, or simply spin) of an electron or other particle. The phrase was originally used to describe ...
(−). The nuclide
A nuclide (or nucleide, from nucleus, also known as nuclear species) is a class of atoms characterized by their number of protons, ''Z'', their number of neutrons, ''N'', and their nuclear energy state.
The word ''nuclide'' was coined by Truma ...
54Fe theoretically can undergo double electron capture
Double electron capture is a decay mode of an atomic nucleus. For a nuclide (''A'', ''Z'') with a number of nucleons ''A'' and atomic number ''Z'', double electron capture is only possible if the mass of the nuclide (''A'', ''Z''−2) is lower.
I ...
to 54Cr, but the process has never been observed and only a lower limit on the half-life of 3.1×1022 years has been established.
60Fe is an extinct radionuclide
An extinct radionuclide is a radionuclide that was formed by nucleosynthesis before the formation of the Solar System, about 4.6 billion years ago, but has since decayed to virtually zero abundance and is no longer detectable as a primordial nuc ...
of long half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable at ...
(2.6 million years). It is not found on Earth, but its ultimate decay product is its granddaughter, the stable nuclide 60Ni. Much of the past work on isotopic composition of iron has focused on the nucleosynthesis
Nucleosynthesis is the process that creates new atomic nuclei from pre-existing nucleons (protons and neutrons) and nuclei. According to current theories, the first nuclei were formed a few minutes after the Big Bang, through nuclear reactions in ...
of 60Fe through studies of meteorite
A meteorite is a solid piece of debris from an object, such as a comet, asteroid, or meteoroid, that originates in outer space and survives its passage through the atmosphere to reach the surface of a planet or moon. When the original object en ...
s and ore formation. In the last decade, advances in mass spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a '' mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is u ...
have allowed the detection and quantification of minute, naturally occurring variations in the ratios of the stable isotope
The term stable isotope has a meaning similar to stable nuclide, but is preferably used when speaking of nuclides of a specific element. Hence, the plural form stable isotopes usually refers to isotopes of the same element. The relative abundanc ...
s of iron. Much of this work is driven by the Earth
Earth is the third planet from the Sun and the only astronomical object known to harbor life. While large volumes of water can be found throughout the Solar System, only Earth sustains liquid surface water. About 71% of Earth's surf ...
and planetary science
Planetary science (or more rarely, planetology) is the scientific study of planets (including Earth), celestial bodies (such as moons, asteroids, comets) and planetary systems (in particular those of the Solar System) and the processes of the ...
communities, although applications to biological and industrial systems are emerging.
In phases of the meteorites ''Semarkona'' and ''Chervony Kut,'' a correlation between the concentration of 60Ni, the granddaughter
Family (from la, familia) is a group of people related either by consanguinity (by recognized birth) or affinity (by marriage or other relationship). The purpose of the family is to maintain the well-being of its members and of society. Ideall ...
of 60Fe, and the abundance of the stable iron isotopes provided evidence for the existence of 60Fe at the time of formation of the Solar System
The formation of the Solar System began about 4.6 billion years ago with the gravitational collapse of a small part of a giant molecular cloud. Most of the collapsing mass collected in the center, forming the Sun, while the rest flattened into a ...
. Possibly the energy released by the decay of 60Fe, along with that released by 26Al, contributed to the remelting and differentiation
Differentiation may refer to:
Business
* Differentiation (economics), the process of making a product different from other similar products
* Product differentiation, in marketing
* Differentiated service, a service that varies with the identity ...
of asteroid
An asteroid is a minor planet of the Solar System#Inner solar system, inner Solar System. Sizes and shapes of asteroids vary significantly, ranging from 1-meter rocks to a dwarf planet almost 1000 km in diameter; they are rocky, metallic o ...
s after their formation 4.6 billion years ago. The abundance of 60Ni present in extraterrestrial
Extraterrestrial refers to any object or being beyond ( extra-) the planet Earth ( terrestrial). It is derived from the Latin words ''extra'' ("outside", "outwards") and ''terrestris'' ("earthly", "of or relating to the Earth"). It may be abbrevia ...
material may bring further insight into the origin and early history of the Solar System
The Solar System Capitalization of the name varies. The International Astronomical Union, the authoritative body regarding astronomical nomenclature, specifies capitalizing the names of all individual astronomical objects but uses mixed "Solar ...
.
The most abundant iron isotope 56Fe is of particular interest to nuclear scientists because it represents the most common endpoint of nucleosynthesis
Nucleosynthesis is the process that creates new atomic nuclei from pre-existing nucleons (protons and neutrons) and nuclei. According to current theories, the first nuclei were formed a few minutes after the Big Bang, through nuclear reactions in ...
. Since 56Ni (14 alpha particle
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay, but may also be pro ...
s) is easily produced from lighter nuclei in the alpha process
The alpha process, also known as the alpha ladder, is one of two classes of nuclear fusion reactions by which stars convert helium into heavier elements, the other being the triple-alpha process.
The triple-alpha process consumes only helium, an ...
in nuclear reaction
In nuclear physics and nuclear chemistry, a nuclear reaction is a process in which two nuclei, or a nucleus and an external subatomic particle, collide to produce one or more new nuclides. Thus, a nuclear reaction must cause a transformatio ...
s in supernovae (see silicon burning process
In astrophysics, silicon burning is a very brief sequence of nuclear fusion reactions that occur in massive stars with a minimum of about 8–11 solar masses. Silicon burning is the final stage of fusion for massive stars that have run out of the ...
), it is the endpoint of fusion chains inside extremely massive stars, since addition of another alpha particle, resulting in 60Zn, requires a great deal more energy. This 56Ni, which has a half-life of about 6 days, is created in quantity in these stars, but soon decays by two successive positron emissions within supernova decay products in the supernova remnant
A supernova remnant (SNR) is the structure resulting from the explosion of a star in a supernova. The supernova remnant is bounded by an expanding shock wave, and consists of ejected material expanding from the explosion, and the interstellar ma ...
gas cloud, first to radioactive 56Co, and then to stable 56Fe. As such, iron is the most abundant element in the core of red giant
A red giant is a luminous giant star of low or intermediate mass (roughly 0.3–8 solar masses ()) in a late phase of stellar evolution. The outer atmosphere is inflated and tenuous, making the radius large and the surface temperature around or ...
s, and is the most abundant metal in iron meteorite
Iron meteorites, also known as siderites, or ferrous meteorites, are a type of meteorite that consist overwhelmingly of an iron–nickel alloy known as meteoric iron that usually consists of two mineral phases: kamacite and taenite. Most i ...
s and in the dense metal cores of planets such as Earth
Earth is the third planet from the Sun and the only astronomical object known to harbor life. While large volumes of water can be found throughout the Solar System, only Earth sustains liquid surface water. About 71% of Earth's surf ...
.Greenwood and Earnshaw, p. 12 It is also very common in the universe, relative to other stable metals
A metal (from Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typicall ...
of approximately the same atomic weight
Relative atomic mass (symbol: ''A''; sometimes abbreviated RAM or r.a.m.), also known by the deprecated synonym atomic weight, is a dimensionless physical quantity defined as the ratio of the average mass of atoms of a chemical element in a give ...
. Iron is the sixth most abundant element in the universe
The universe is all of space and time and their contents, including planets, stars, galaxies, and all other forms of matter and energy. The Big Bang theory is the prevailing cosmological description of the development of the universe. A ...
, and the most common refractory
In materials science, a refractory material or refractory is a material that is resistant to decomposition by heat, pressure, or chemical attack, and retains strength and form at high temperatures. Refractories are polycrystalline, polyphase ...
element.
Although a further tiny energy gain could be extracted by synthesizing 62Ni, which has a marginally higher binding energy than 56Fe, conditions in stars are unsuitable for this process. Element production in supernovas and distribution on Earth greatly favor iron over nickel, and in any case, 56Fe still has a lower mass per nucleon than 62Ni due to its higher fraction of lighter protons. Hence, elements heavier than iron require a supernova for their formation, involving rapid neutron capture by starting 56Fe nuclei.
In the far future
While the future cannot be predicted with certainty, present understanding in various scientific fields allows for the prediction of some far-future events, if only in the broadest outline. These fields include astrophysics, which studies how ...
of the universe, assuming that proton decay
In particle physics, proton decay is a hypothetical form of particle decay in which the proton decays into lighter subatomic particles, such as a neutral pion and a positron. The proton decay hypothesis was first formulated by Andrei Sakha ...
does not occur, cold fusion occurring via quantum tunnelling
Quantum tunnelling, also known as tunneling ( US) is a quantum mechanical phenomenon whereby a wavefunction can propagate through a potential barrier.
The transmission through the barrier can be finite and depends exponentially on the barrier ...
would cause the light nuclei in ordinary matter to fuse into 56Fe nuclei. Fission and alpha-particle emission would then make heavy nuclei decay into iron, converting all stellar-mass objects to cold spheres of pure iron.
Origin and occurrence in nature
Cosmogenesis
Iron's abundance in rocky planets like Earth is due to its abundant production during the runaway fusion and explosion of type Ia supernovae, which scatters the iron into space.Metallic iron
 Metallic or
Metallic or native iron
Telluric iron, also called native iron, is iron that originated on Earth, and is found in a metallic form rather than as an ore. Telluric iron is extremely rare, with only one known major deposit in the world, located in Greenland.
Introduction
W ...
is rarely found on the surface of the Earth because it tends to oxidize. However, both the Earth's inner and outer core
Earth's outer core is a fluid layer about thick, composed of mostly iron and nickel that lies above Earth's solid inner core and below its mantle. The outer core begins approximately beneath Earth's surface at the core-mantle boundary and en ...
, that account for 35% of the mass of the whole Earth, are believed to consist largely of an iron alloy, possibly with nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow ...
. Electric currents in the liquid outer core are believed to be the origin of the Earth's magnetic field
Earth's magnetic field, also known as the geomagnetic field, is the magnetic field that extends from Earth's interior out into space, where it interacts with the solar wind, a stream of charged particles emanating from the Sun. The magneti ...
. The other terrestrial planet
A terrestrial planet, telluric planet, or rocky planet, is a planet that is composed primarily of silicate rocks or metals. Within the Solar System, the terrestrial planets accepted by the IAU are the inner planets closest to the Sun: Mercury, ...
s ( Mercury, Venus
Venus is the second planet from the Sun. It is sometimes called Earth's "sister" or "twin" planet as it is almost as large and has a similar composition. As an interior planet to Earth, Venus (like Mercury) appears in Earth's sky never f ...
, and Mars
Mars is the fourth planet from the Sun and the second-smallest planet in the Solar System, only being larger than Mercury. In the English language, Mars is named for the Roman god of war. Mars is a terrestrial planet with a thin atmos ...
) as well as the Moon
The Moon is Earth's only natural satellite. It is the fifth largest satellite in the Solar System and the largest and most massive relative to its parent planet, with a diameter about one-quarter that of Earth (comparable to the width ...
are believed to have a metallic core consisting mostly of iron. The M-type asteroid
M-type (aka M-class) asteroids are a spectral class of asteroids which appear to contain higher concentrations of metal phases (e.g. iron-nickel) than other asteroid classes, and are widely thought to be the source of iron meteorites.
Definition ...
s are also believed to be partly or mostly made of metallic iron alloy.
The rare iron meteorite
Iron meteorites, also known as siderites, or ferrous meteorites, are a type of meteorite that consist overwhelmingly of an iron–nickel alloy known as meteoric iron that usually consists of two mineral phases: kamacite and taenite. Most i ...
s are the main form of natural metallic iron on the Earth's surface. Items made of cold-worked meteoritic iron have been found in various archaeological sites dating from a time when iron smelting had not yet been developed; and the Inuit
Inuit (; iu, ᐃᓄᐃᑦ 'the people', singular: Inuk, , dual: Inuuk, ) are a group of culturally similar indigenous peoples inhabiting the Arctic and subarctic regions of Greenland, Labrador, Quebec, Nunavut, the Northwest Territories, ...
in Greenland
Greenland ( kl, Kalaallit Nunaat, ; da, Grønland, ) is an island country in North America that is part of the Kingdom of Denmark. It is located between the Arctic and Atlantic oceans, east of the Canadian Arctic Archipelago. Greenland is ...
have been reported to use iron from the Cape York meteorite
The Cape York meteorite, also known as the Innaanganeq meteorite, is one of the largest known iron meteorites, classified as a medium octahedrite in chemical group IIIAB. In addition to many small fragments, at least eight large fragments with a ...
for tools and hunting weapons. About 1 in 20 meteorite
A meteorite is a solid piece of debris from an object, such as a comet, asteroid, or meteoroid, that originates in outer space and survives its passage through the atmosphere to reach the surface of a planet or moon. When the original object en ...
s consist of the unique iron-nickel minerals taenite
Taenite is a mineral found naturally on Earth mostly in iron meteorites. It is an alloy of iron and nickel, with a chemical formula of and nickel proportions of 20% up to 65%.
The name is derived from the Greek ταινία for "band, ribbon" ...
(35–80% iron) and kamacite
Kamacite is an alloy of iron and nickel, which is found on Earth only in meteorites. According to the International Mineralogical Association (IMA) it is considered a proper nickel-rich variety of the mineral native iron. The proportion iron:ni ...
(90–95% iron). Native iron is also rarely found in basalts that have formed from magmas that have come into contact with carbon-rich sedimentary rocks, which have reduced the oxygen fugacity
In chemical thermodynamics, the fugacity of a real gas is an effective partial pressure which replaces the mechanical partial pressure in an accurate computation of the chemical equilibrium constant. It is equal to the pressure of an ideal gas ...
sufficiently for iron to crystallize. This is known as Telluric iron
Telluric iron, also called native iron, is iron that originated on Earth, and is found in a metallic form rather than as an ore. Telluric iron is extremely rare, with only one known major deposit in the world, located in Greenland.
Introduction
Wi ...
and is described from a few localities, such as Disko Island
Disko Island ( kl, Qeqertarsuaq, da, Diskoøen) is a large island in Baffin Bay, off the west coast of Greenland. It has an area of ,Yakutia
Sakha, officially the Republic of Sakha (Yakutia),, is the largest republic of Russia, located in the Russian Far East, along the Arctic Ocean, with a population of roughly 1 million. Sakha comprises half of the area of its governing Far ...
in Russia and Bühl in Germany.
Mantle minerals
Ferropericlase
Ferropericlase or magnesiowüstite is a magnesium/iron oxide with the chemical formula that is interpreted to be one of the main constituents of the Earth's lower mantle together with the silicate perovskite (), a magnesium/iron silicate with a ...
, a solid solution of periclase
Periclase is a magnesium mineral that occurs naturally in contact metamorphic rocks and is a major component of most basic refractory bricks. It is a cubic form of magnesium oxide ( Mg O). In nature it usually forms a solid solution with wüsti ...
(MgO) and wüstite
Wüstite ( Fe O) is a mineral form of iron(II) oxide found with meteorites and native iron. It has a grey colour with a greenish tint in reflected light. Wüstite crystallizes in the isometric-hexoctahedral crystal system in opaque to translu ...
(FeO), makes up about 20% of the volume of the lower mantle
The lower mantle, historically also known as the mesosphere, represents approximately 56% of Earth's total volume, and is the region from 660 to 2900 km below Earth's surface; between the transition zone and the outer core. The preliminary ...
of the Earth, which makes it the second most abundant mineral phase in that region after silicate perovskite
Silicate perovskite is either (the magnesium end-member is called bridgmanite) or (calcium silicate known as davemaoite) when arranged in a perovskite structure. Silicate perovskites are not stable at Earth's surface, and mainly exist in the l ...
; it also is the major host for iron in the lower mantle. At the bottom of the transition zone of the mantle, the reaction γ- transforms γ-olivine into a mixture of silicate perovskite and ferropericlase and vice versa. In the literature, this mineral phase of the lower mantle is also often called magnesiowüstite.FerropericlaseMindat.org
Silicate perovskite
Silicate perovskite is either (the magnesium end-member is called bridgmanite) or (calcium silicate known as davemaoite) when arranged in a perovskite structure. Silicate perovskites are not stable at Earth's surface, and mainly exist in the l ...
may form up to 93% of the lower mantle, and the magnesium iron form, , is considered to be the most abundant mineral
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid chemical compound with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.John P. Rafferty, ed. ...
in the Earth, making up 38% of its volume.
Earth's crust
outer
{{Short pages monitor
Building materials
Pyrotechnic fuels
Chemical elements with body-centered cubic structure
Native element minerals