|
Histidine
Histidine (symbol His or H) is an essential amino acid that is used in the biosynthesis of proteins. It contains an Amine, α-amino group (which is in the protonated –NH3+ form under Physiological condition, biological conditions), a carboxylic acid group (which is in the deprotonated –COO− form under biological conditions), and an imidazole side chain (which is partially protonated), classifying it as a positively charged amino acid at physiological pH. Initially thought essential amino acid, essential only for infants, it has now been shown in longer-term studies to be essential for adults also. It is Genetic code, encoded by the Genetic code, codons CAU and CAC. Histidine was first isolated by Albrecht Kossel and Sven Gustaf Hedin in 1896. The name stems from its discovery in tissue, from ''histós'' "tissue". It is also a Precursor (chemistry), precursor to histamine, a vital inflammatory agent in immune responses. The acyl radical (chemistry), radical is histidyl. Pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Essential Amino Acid
An essential amino acid, or indispensable amino acid, is an amino acid that cannot be synthesized from scratch by the organism fast enough to supply its demand, and must therefore come from the diet. Of the 21 amino acids common to all life forms, the nine amino acids humans cannot synthesize are valine, isoleucine, leucine, methionine, phenylalanine, tryptophan, threonine, histidine, and lysine. Six other amino acids are considered conditionally essential in the human diet, meaning their synthesis can be limited under special pathophysiological conditions, such as prematurity in the infant or individuals in severe catabolic distress. These six are arginine, cysteine, glycine, glutamine, proline, and tyrosine. Six amino acids are non-essential (dispensable) in humans, meaning they can be synthesized in sufficient quantities in the body. These six are alanine, aspartic acid, asparagine, glutamic acid, serine, and selenocysteine (considered the 21st amino acid). Pyrr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalytic Triad
A catalytic triad is a set of three coordinated amino acid residues that can be found in the active site of some enzymes. Catalytic triads are most commonly found in hydrolase and transferase enzymes (e.g. proteases, amidases, esterases, aminoacylase, acylases, lipases and β-lactamases). An acid-base (chemistry), base-nucleophile triad is a common motif for generating a nucleophilic residue for covalent catalysis. The residues form a charge-relay network to polarise and activate the nucleophile, which attacks the Substrate (chemistry), substrate, forming a covalent intermediate which is then hydrolysed to release the Product (chemistry), product and regenerate free enzyme. The nucleophile is most commonly a serine or cysteine, but occasionally threonine or even selenocysteine. The Protein tertiary structure, 3D structure of the enzyme brings together the triad residues in a precise orientation, even though they may be far apart in the sequence (Protein primary structure, primary ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Imidazole
Imidazole (ImH) is an organic compound with the formula . It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. It can be classified as a heterocycle, specifically as a diazole. Many natural products, especially alkaloids, contain the imidazole ring. These imidazoles share the 1,3-C3N2 ring but feature varied substituents. This ring system is present in important biological building blocks, such as histidine and the related hormone histamine. Many drugs contain an imidazole ring, such as certain antifungal drugs, the nitroimidazole series of antibiotics, and the sedative midazolam. When fused to a pyrimidine ring, it forms a purine, which is the most widely occurring nitrogen-containing Heterocyclic compound, heterocycle in nature. The name "imidazole" was coined in 1887 by the German chemist Arthur Rudolf Hantzsch (1857–1935). Structure and properties Imidazole is a planar 5-membered ring, that exists in two equivalent tautomeric f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Histamine
Histamine is an organic nitrogenous compound involved in local immune responses communication, as well as regulating physiological functions in the gut and acting as a neurotransmitter for the brain, spinal cord, and uterus. Discovered in 1910, histamine has been considered a local hormone ( autocoid) because it is produced without involvement of the classic endocrine glands; however, in recent years, histamine has been recognized as a central neurotransmitter. Histamine is involved in the inflammatory response and has a central role as a mediator of itching. As part of an immune response to foreign pathogens, histamine is produced by basophils and by mast cells found in nearby connective tissues. Histamine increases the permeability of the capillaries to white blood cells and some proteins, to allow them to engage pathogens in the infected tissues. It consists of an imidazole ring attached to an ethylamine chain; under physiological conditions, the amino grou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sven Gustaf Hedin
Sven Gustaf Hedin (6 October 1859 – 11 July 1933) was a Swedish chemist and physiologist credited with the discovery of histidine. He was born in Alseda parish, Jönköping County. He began his studies in 1878 and received his bachelor's degree in 1881 at Uppsala University. In 1886 he received his doctorate in philosophy and doctorate of medicine in 1893 in Lund and docent in chemistry in 1886 and became a researcher () there in 1895. From 1900 to 1907 he was made head of the pathological chemistry division at the Lister Institute of Preventive Medicine in London, and professor of medicinal and physiological chemistry at Uppsala University in 1908. Hedin was a member of the Royal Physiographic Society in Lund (1896) and the Royal Swedish Academy of Sciences (1902). Among Hedin's many scientific theses are (1886) and (1893, both in Lund University's journal), (in ''Zeitschrift für physiologische Chemie'', 1895) and other reports on the decomposition of protein in the same ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ball-and-stick Model
In chemistry, the ball-and-stick model is a molecular model of a chemical substance which displays both the Molecular geometry, three-dimensional position of the atoms and the chemical bond, bonds between them. The atoms are typically represented by sphere (geometry), spheres, connected by rods which represent the bonds. Double bond, Double and triple bonds are usually represented by two or three curved rods, respectively, or alternately by correctly positioned sticks for the sigma bond, sigma and pi bonds. In a good model, the angles between the rods should be the same as the Bond angle, angles between the bonds, and the distances between the centers of the spheres should be proportional to the distances between the corresponding atomic nucleus, atomic nuclei. The chemical element of each atom is often indicated by the sphere's color. In a ball-and-stick model, the radius of the spheres is usually much smaller than the rod lengths, in order to provide a clearer view of the atoms ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Henderson–Hasselbalch Equation
In chemistry and biochemistry, the pH of weakly acidic Solution (chemistry), chemical solutions can be estimated using the Henderson-Hasselbach Equation: \ce = \ceK_\ce + \log_ \left( \frac \right) The equation relates the pH of the weak acid to the numerical value of the acid dissociation constant, ''K''a, of the acid strength, acid, and the ratio of the concentrations of the acid and its Conjugate (acid-base theory), conjugate base. ''Acid-base Equilibrium Reaction'' \mathrm The Henderson-Hasselbalch equation is often used for estimating the pH of buffer solutions by approximating the actual concentration ratio as the ratio of the analytical concentrations of the acid and of a salt, MA. It is also useful for determining the volumes of the reagents needed before preparing buffer solutions, which prevents unncessary waste of chemical reagents that may need to be further neutralized by even more reagents before they are safe to expose. For example, the acid may be carbo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. It is a common element in the universe, estimated at Abundance of the chemical elements, seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element chemical bond, bond to form N2, a colourless and odourless diatomic molecule, diatomic gas. N2 forms about 78% of Atmosphere of Earth, Earth's atmosphere, making it the most abundant chemical species in air. Because of the volatility of nitrogen compounds, nitrogen is relatively rare in the solid parts of the Earth. It was first discovered and isolated by Scottish physician Daniel Rutherford in 1772 and independently by Carl Wilhelm Scheele and Henry Cavendish at about the same time. The name was suggested by French chemist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
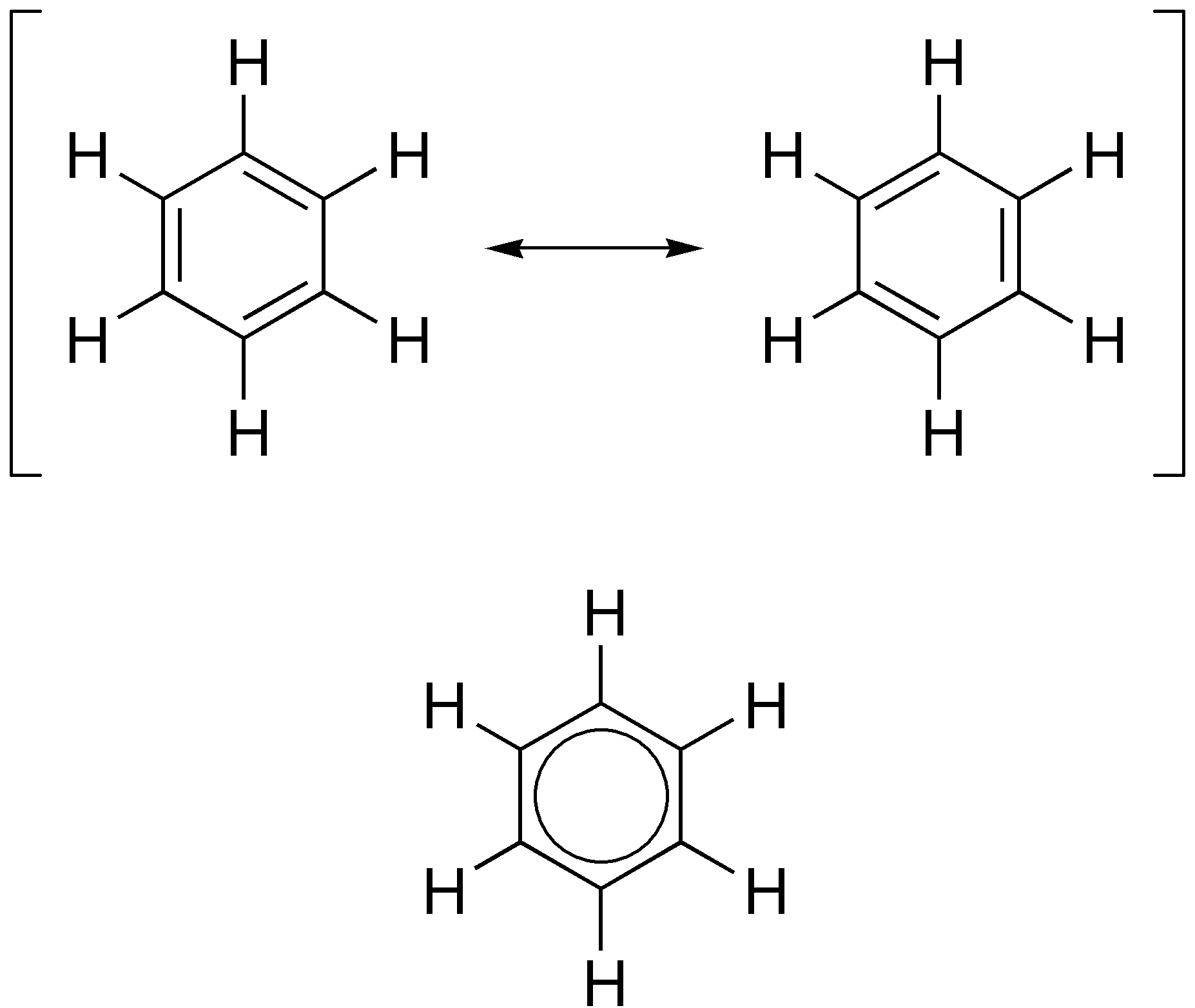
Resonance Structure
In chemistry, resonance, also called mesomerism, is a way of describing bonding in certain molecules or polyatomic ions by the combination of several contributing structures (or ''forms'', also variously known as ''resonance structures'' or ''canonical structures'') into a resonance hybrid (or ''hybrid structure'') in valence bond theory. It has particular value for analyzing delocalized electrons where the bonding cannot be expressed by one single Lewis structure. The resonance hybrid is the accurate structure for a molecule or ion; it is an average of the theoretical (or hypothetical) contributing structures. Overview Under the framework of valence bond theory, resonance is an extension of the idea that the bonding in a chemical species can be described by a Lewis structure. For many chemical species, a single Lewis structure, consisting of atoms obeying the octet rule, possibly bearing formal charges, and connected by bonds of positive integer order, is sufficient for des ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aromatic
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated system, conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugation alone. The earliest use of the term was in an article by August Wilhelm Hofmann in 1855. There is no general relationship between aromaticity as a chemical property and the olfaction, olfactory properties of such compounds. Aromaticity can also be considered a manifestation of cyclic delocalization and of Resonance (chemistry), resonance. This is usually considered to be because electrons are free to cycle around circular arrangements of atoms that are alternately single- and double-covalent bond, bonded to one another. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by Friedrich August Kekulé ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tautomer
In chemistry, tautomers () are structural isomers (constitutional isomers) of chemical compounds that readily interconvert. The chemical reaction interconverting the two is called tautomerization. This conversion commonly results from the relocation of a hydrogen atom within the compound. The phenomenon of tautomerization is called tautomerism, also called desmotropism. Tautomerism is for example relevant to the behavior of amino acids and nucleic acids, two of the fundamental building blocks of life. Care should be taken not to confuse tautomers with depictions of "contributing structures" in chemical resonance. Tautomers are distinct chemical species that can be distinguished by their differing atomic connectivities, molecular geometries, and physicochemical and spectroscopic properties, whereas resonance forms are merely alternative Lewis structure (valence bond theory) depictions of a single chemical species, whose true structure is a quantum superposition, essentially the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acid Dissociation Constant
In chemistry, an acid dissociation constant (also known as acidity constant, or acid-ionization constant; denoted ) is a quantitative property, quantitative measure of the acid strength, strength of an acid in Solution (chemistry), solution. It is the equilibrium constant for a chemical reaction :HA A^- + H^+ known as Dissociation (chemistry), dissociation in the context of acid–base reactions. The chemical species HA is an acid that dissociates into , called the conjugate base of the acid, and a hydron (chemistry), hydrogen ion, . The system is said to be in chemical equilibrium, equilibrium when the concentrations of its components do not change over time, because both forward and backward reactions are occurring at the same rate. The dissociation constant is defined by :K_\text = \mathrm, or by its logarithmic form :\mathrmK_\ce = - \log_ K_\text = \log_\frac where quantities in square brackets represent the molar concentrations of the species at equilibrium. For example ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |