|
Iron-56
Iron-56 (56Fe) is the most common isotope of iron. About 91.754% of all iron is iron-56. Of all nuclides, iron-56 has the lowest mass per nucleon. With 8.8 MeV binding energy per nucleon, iron-56 is one of the most tightly bound nuclei. The high nuclear binding energy for 56Fe represents the point where further nuclear reactions become energetically unfavorable. Because of this, it is among the heaviest elements formed in stellar nucleosynthesis reactions in massive stars. These reactions fuse lighter elements like magnesium, silicon, and sulfur to form heavier elements. Among the heavier elements formed is 56Ni, which subsequently decays to 56Co and then 56Fe. Relationship to nickel-62 Nickel-62, a relatively rare isotope of nickel, has a higher nuclear binding energy per nucleon; this is consistent with having a higher mass-per-nucleon because nickel-62 has a greater proportion of neutrons, which are slightly more massive than protons. (See the nickel-62 article ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Binding Energy
Nuclear binding energy in experimental physics is the minimum energy that is required to disassemble the nucleus of an atom into its constituent protons and neutrons, known collectively as nucleons. The binding energy for stable nuclei is always a positive number, as the nucleus must gain energy for the nucleons to move apart from each other. Nucleons are attracted to each other by the strong nuclear force. In theoretical nuclear physics, the nuclear binding energy is considered a negative number. In this context it represents the energy of the nucleus relative to the energy of the constituent nucleons when they are infinitely far apart. Both the experimental and theoretical views are equivalent, with slightly different emphasis on what the binding energy means. The mass of an atomic nucleus is less than the sum of the individual masses of the free constituent protons and neutrons. The difference in mass can be calculated by the Einstein equation, , where ''E'' is the nuclea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nickel-62
Nickel-62 is an isotope of nickel having 28 protons and 34 neutrons. It is a stable isotope, with the highest binding energy per nucleon of any known nuclide (8.7945 MeV). It is often stated that 56Fe is the "most stable nucleus", but only because 56Fe has the lowest ''mass'' per nucleon (not binding energy per nucleon) of all nuclides. The lower mass per nucleon of 56Fe is possible because 56Fe has 26/56 ≈ 46.43% protons, while 62Ni has only 28/62 ≈ 45.16% protons. Protons are less massive than neutrons, meaning that the larger fraction of protons in 56Fe lowers its mean mass-per-nucleon ratio in a way that has no effect on its binding energy. In other words, Nickel-62 still has the least massive protons and neutrons of any isotope. Properties The high binding energy of nickel isotopes in general makes nickel an "end product" of many nuclear reactions (including neutron capture reactions) throughout the universe and accounts for the high relative abundance of nickel—al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron Star
In astronomy, the term iron star has been used for two unrelated types of star: * a blue supergiant with a forest of forbidden FeII lines in its spectrum. * a hypothetical type of compact star. Blue supergiant An iron star is a type of blue supergiant which has a forest of forbidden FeII lines in its spectrum. They are potentially quiescent hot luminous blue variables. Eta Carinae has been described as a prototypical example. Compact iron star formation An iron star is a hypothetical type of compact star that could occur in the universe in the extremely far future, after perhaps 101500 years. The premise behind the formation of iron stars states that cold fusion occurring via quantum tunnelling would cause the light nuclei in ordinary matter to fuse into iron-56 nuclei, due to its nature as the atomic nucleus with the lowest mass per nucleon. Fission and alpha-particle emission would then make heavy nuclei decay into iron, converting stellar-mass objects to cold spheres of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's outer and inner core. It is the fourth most abundant element in the Earth's crust, being mainly deposited by meteorites in its metallic state. Extracting usable metal from iron ores requires kilns or furnaces capable of reaching , about 500 °C (900 °F) higher than that required to smelt copper. Humans started to master that process in Eurasia during the 2nd millennium BC and the use of iron tools and weapons began to displace copper alloys – in some regions, only around 1200 BC. That event is considered the transition from the Bronze Age to the Iron Age. In the modern world, iron alloys, such as steel, stainless steel, cast iron and special steels, are by far the most common industrial metals, due to their mechan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron Star
In astronomy, the term iron star has been used for two unrelated types of star: * a blue supergiant with a forest of forbidden FeII lines in its spectrum. * a hypothetical type of compact star. Blue supergiant An iron star is a type of blue supergiant which has a forest of forbidden FeII lines in its spectrum. They are potentially quiescent hot luminous blue variables. Eta Carinae has been described as a prototypical example. Compact iron star formation An iron star is a hypothetical type of compact star that could occur in the universe in the extremely far future, after perhaps 101500 years. The premise behind the formation of iron stars states that cold fusion occurring via quantum tunnelling would cause the light nuclei in ordinary matter to fuse into iron-56 nuclei, due to its nature as the atomic nucleus with the lowest mass per nucleon. Fission and alpha-particle emission would then make heavy nuclei decay into iron, converting stellar-mass objects to cold spheres of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Fusion
Nuclear fusion is a nuclear reaction, reaction in which two or more atomic nuclei combine to form a larger nuclei, nuclei/neutrons, neutron by-products. The difference in mass between the reactants and products is manifested as either the release or absorption (electromagnetic radiation), absorption of energy. This difference in mass arises as a result of the difference in nuclear binding energy between the atomic nuclei before and after the fusion reaction. Nuclear fusion is the process that powers all active stars, via many Stellar nucleosynthesis, reaction pathways. Fusion processes require an extremely large Lawson criterion, triple product of temperature, density, and confinement time. These conditions occur only in Stellar core, stellar cores, advanced Nuclear weapon design, nuclear weapons, and are approached in List of fusion experiments, fusion power experiments. A nuclear fusion process that produces atomic nuclei lighter than nickel-62 is generally exothermic, due t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron-55
Iron-55 (55Fe) is a radioactive isotope of iron with a nucleus containing 26 protons and 29 neutrons. It decays by electron capture to manganese-55 and this process has a half-life of 2.737 years. The emitted X-rays can be used as an X-ray source for various scientific analysis methods, such as X-ray diffraction. Iron-55 is also a source for Auger electrons, which are produced during the decay. Decay Iron-55 decays via electron capture to manganese-55 with a half-life of 2.737 years. The electrons around the nucleus rapidly adjust themselves to the lowered charge without leaving their shell, and shortly thereafter the vacancy in the "K" shell left by the nuclear-captured electron is filled by an electron from a higher shell. The difference in energy is released by emitting Auger electrons of 5.19 keV, with a probability of about 60%, K-alpha-1 X-rays with energy of 5.89875 keV and a probability about 16.2%, K-alpha-2 X-rays with energy of 5.88765 keV and a probabil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Future Of An Expanding Universe
Current observations suggest that the expansion of the universe will continue forever. The prevailing theory is that the universe will cool as it expands, eventually becoming too cold to sustain life. For this reason, this future scenario popularly called " Heat Death" is also known as the "Big Chill" or "Big Freeze". Some of the other popular theories include the Big Rip, Big Crunch, and the Big Bounce. If dark energy—represented by the cosmological constant, a ''constant'' energy density filling space homogeneously, or scalar fields, such as quintessence or moduli, ''dynamic'' quantities whose energy density can vary in time and space—accelerates the expansion of the universe, then the space between clusters of galaxies will grow at an increasing rate. Redshift will stretch ancient ambient photons (including gamma rays) to undetectably long wavelengths and low energies. Stars are expected to form normally for 1012 to 1014 (1–100 trillion) years, but eventually th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
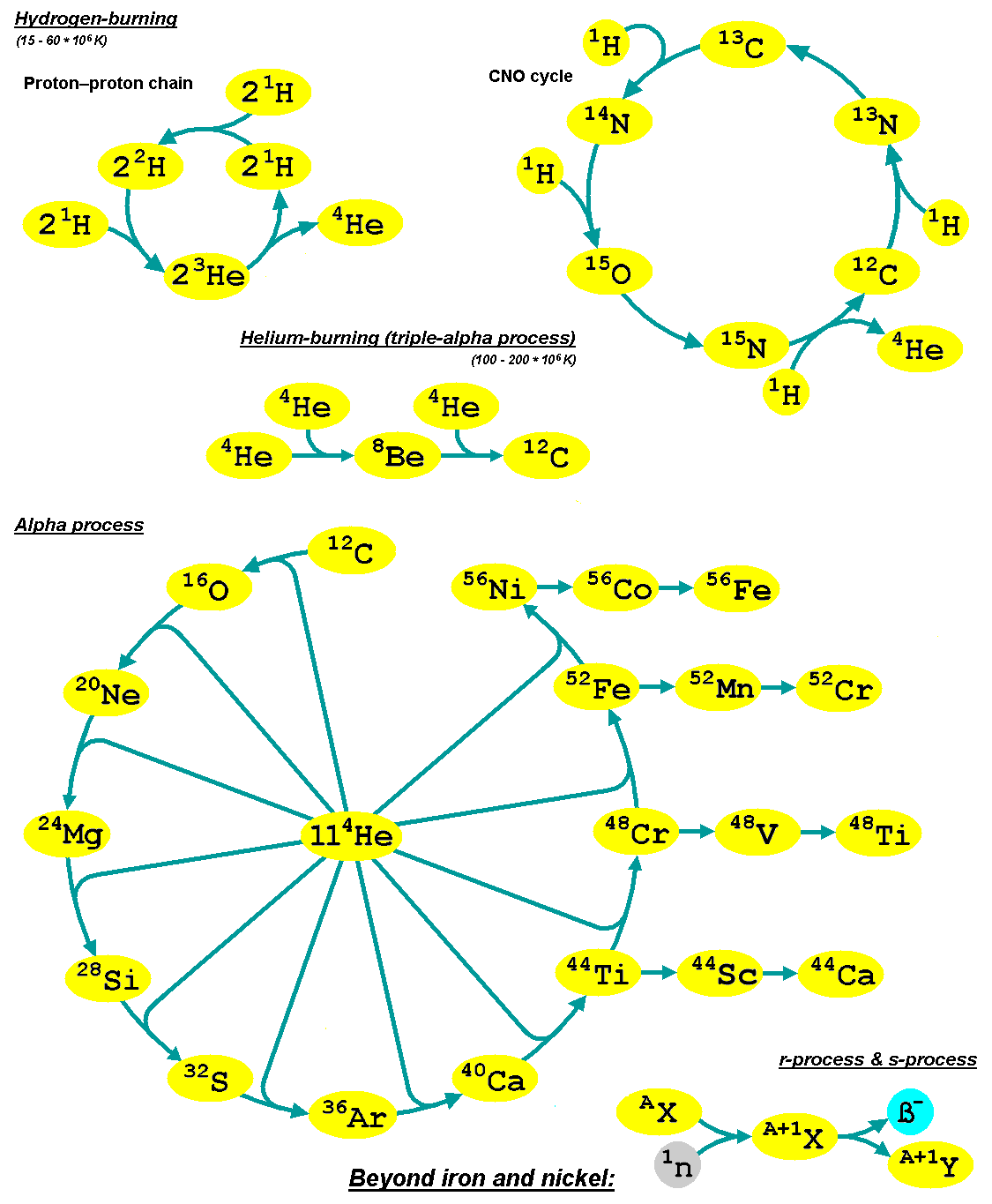
Alpha Process
The alpha process, also known as alpha capture or the alpha ladder, is one of two classes of nuclear fusion reactions by which stars convert helium into heavier elements. The other class is a cycle of reactions called the triple-alpha process, which consumes only helium, and produces carbon. The alpha process most commonly occurs in massive stars and during supernovae. Both processes are preceded by hydrogen fusion, which produces the helium that fuels both the triple-alpha process and the alpha ladder processes. After the triple-alpha process has produced enough carbon, the alpha-ladder begins and fusion reactions of increasingly heavy elements take place, in the order listed below. Each step only consumes the product of the previous reaction and helium. The later-stage reactions which are able to begin in any particular star, do so while the prior stage reactions are still under way in outer layers of the star. :\begin \ce& E=\mathsf \\ \ce& E=\mathsf \\ \ce& E=\mathsf \\ \c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Manganese-56
Naturally occurring manganese (25Mn) is composed of one stable isotope, 55Mn. Twenty-seven radioisotopes have been characterized, with the most stable being 53Mn with a half-life of 3.7 million years, 54Mn with a half-life of 312.3 days, and 52Mn with a half-life of 5.591 days. All of the remaining radioactive isotopes have half-lives that are less than 3 hours and the majority of these have half-lives that are less than a minute. This element also has seven meta states. Manganese is part of the iron group of elements, which are thought to be synthesized in large stars shortly before supernova explosions. 53Mn decays to 53 Cr with a half-life of 3.7 million years. Because of its relatively short half-life, 53Mn occurs only in tiny amounts due to the action of cosmic rays on iron in rocks. Manganese isotopic contents are typically combined with chromium isotopic contents and have found application in isotope geology and radiometric dating. Mn−Cr isotopic ratios reinforce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron-57
Natural iron (Fe) consists of four stable isotopes: 5.845% Fe (possibly radioactive with half-life > years), 91.754% Fe, 2.119% Fe and 0.286% Fe. There are 28 known radioisotopes and 8 nuclear isomers, the most stable of which are Fe (half-life 2.6 million years) and Fe (half-life 2.7 years). Much of the past work on measuring the isotopic composition of iron has centered on determining Fe variations due to processes accompanying nucleosynthesis (i.e., meteorite studies) and ore formation. In the last decade however, advances in mass spectrometry technology have allowed the detection and quantification of minute, naturally occurring variations in the ratios of the stable isotopes of iron. Much of this work has been driven by the Earth and planetary science communities, though applications to biological and industrial systems are beginning to emerge. List of isotopes , -id=Iron-45 , rowspan=4, 45Fe , rowspan=4 style="text-align:right" , 26 , rowspan=4 style="text-al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |