|
Nickel-62
Nickel-62 is an isotope of nickel having 28 protons and 34 neutrons. It is a stable isotope, with the highest binding energy per nucleon of any known nuclide (8.7945 MeV). It is often stated that 56Fe is the "most stable nucleus", but only because 56Fe has the lowest ''mass'' per nucleon (not binding energy per nucleon) of all nuclides. The lower mass per nucleon of 56Fe is possible because 56Fe has 26/56 ≈ 46.43% protons, while 62Ni has only 28/62 ≈ 45.16% protons. Protons are less massive than neutrons, meaning that the larger fraction of protons in 56Fe lowers its mean mass-per-nucleon ratio in a way that has no effect on its binding energy. In other words, Nickel-62 still has the least massive protons and neutrons of any isotope. Properties The high binding energy of nickel isotopes in general makes nickel an "end product" of many nuclear reactions (including neutron capture reactions) throughout the universe and accounts for the high relative abundance of nickel—al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopes Of Nickel
Naturally occurring nickel (Ni) consists of five stable isotopes; Ni, Ni, Ni, Ni and Ni; Ni is the most abundant (68.077% natural abundance). 26 radioisotopes have been characterized; the most stable are Ni with a half-life of 81,000 years, Ni with a half-life of 100.1 years, and Ni (6.077 days). All the other radioactive isotopes have half-lives of less than 60 hours and most of these have half-lives of less than 30 seconds. This element also has 8 meta states. List of isotopes , - , rowspan=3, , rowspan=3 style="text-align:right" , 28 , rowspan=3 style="text-align:right" , 20 , rowspan=3, 48.01952(46)# , rowspan=3, 2.8(8) ms , 2 p (70%) , , rowspan=3, 0+ , rowspan=3, , rowspan=3, , - , β+ (30%) , , - , β+, p? , , -id=Nickel-49 , rowspan=2, , rowspan=2 style="text-align:right" , 28 , rowspan=2 style="text-align:right" , 21 , rowspan=2, 49.00916(64)# , rowspan=2, 7.5(10) ms , β+, p (83%) , , rowspan=2, 7/2−# , rowspan=2, , rowspan=2, , - , ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nickel-58
Naturally occurring nickel (Ni) consists of five stable isotopes; Ni, Ni, Ni, Ni and Ni; Ni is the most abundant (68.077% natural abundance). 26 radioisotopes have been characterized; the most stable are Ni with a half-life of 81,000 years, Ni with a half-life of 100.1 years, and Ni (6.077 days). All the other radioactive isotopes have half-lives of less than 60 hours and most of these have half-lives of less than 30 seconds. This element also has 8 meta states. List of isotopes , - , rowspan=3, , rowspan=3 style="text-align:right" , 28 , rowspan=3 style="text-align:right" , 20 , rowspan=3, 48.01952(46)# , rowspan=3, 2.8(8) ms , 2 p (70%) , , rowspan=3, 0+ , rowspan=3, , rowspan=3, , - , β+ (30%) , , - , β+, p? , , -id=Nickel-49 , rowspan=2, , rowspan=2 style="text-align:right" , 28 , rowspan=2 style="text-align:right" , 21 , rowspan=2, 49.00916(64)# , rowspan=2, 7.5(10) ms , β+, p (83%) , , rowspan=2, 7/2−# , rowspan=2, , rowspan=2, , - , β ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Binding Energy
Nuclear binding energy in experimental physics is the minimum energy that is required to disassemble the nucleus of an atom into its constituent protons and neutrons, known collectively as nucleons. The binding energy for stable nuclei is always a positive number, as the nucleus must gain energy for the nucleons to move apart from each other. Nucleons are attracted to each other by the strong nuclear force. In theoretical nuclear physics, the nuclear binding energy is considered a negative number. In this context it represents the energy of the nucleus relative to the energy of the constituent nucleons when they are infinitely far apart. Both the experimental and theoretical views are equivalent, with slightly different emphasis on what the binding energy means. The mass of an atomic nucleus is less than the sum of the individual masses of the free constituent protons and neutrons. The difference in mass can be calculated by the Einstein equation, , where ''E'' is the nuclea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nickel-61
Naturally occurring nickel (Ni) consists of five stable isotopes; Ni, Ni, Ni, Ni and Ni; Ni is the most abundant (68.077% natural abundance). 26 radioisotopes have been characterized; the most stable are Ni with a half-life of 81,000 years, Ni with a half-life of 100.1 years, and Ni (6.077 days). All the other radioactive isotopes have half-lives of less than 60 hours and most of these have half-lives of less than 30 seconds. This element also has 8 meta states. List of isotopes , - , rowspan=3, , rowspan=3 style="text-align:right" , 28 , rowspan=3 style="text-align:right" , 20 , rowspan=3, 48.01952(46)# , rowspan=3, 2.8(8) ms , 2 p (70%) , , rowspan=3, 0+ , rowspan=3, , rowspan=3, , - , β+ (30%) , , - , β+, p? , , -id=Nickel-49 , rowspan=2, , rowspan=2 style="text-align:right" , 28 , rowspan=2 style="text-align:right" , 21 , rowspan=2, 49.00916(64)# , rowspan=2, 7.5(10) ms , β+, p (83%) , , rowspan=2, 7/2−# , rowspan=2, , rowspan=2, , - , β ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
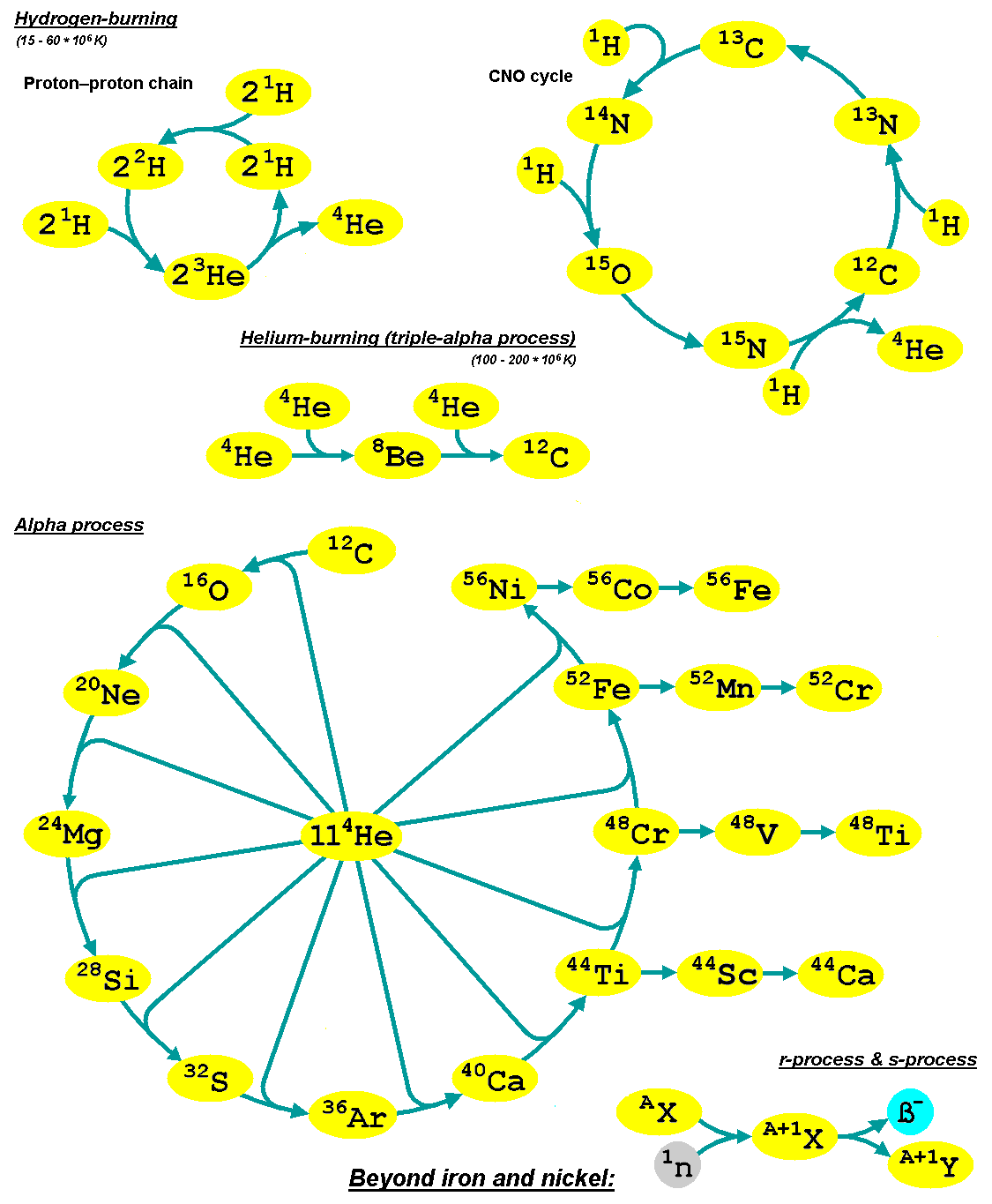
Alpha Process
The alpha process, also known as alpha capture or the alpha ladder, is one of two classes of nuclear fusion reactions by which stars convert helium into heavier elements. The other class is a cycle of reactions called the triple-alpha process, which consumes only helium, and produces carbon. The alpha process most commonly occurs in massive stars and during supernovae. Both processes are preceded by hydrogen fusion, which produces the helium that fuels both the triple-alpha process and the alpha ladder processes. After the triple-alpha process has produced enough carbon, the alpha-ladder begins and fusion reactions of increasingly heavy elements take place, in the order listed below. Each step only consumes the product of the previous reaction and helium. The later-stage reactions which are able to begin in any particular star, do so while the prior stage reactions are still under way in outer layers of the star. :\begin \ce& E=\mathsf \\ \ce& E=\mathsf \\ \ce& E=\mathsf \\ \c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stable Nuclide
Stable nuclides are isotopes of a chemical element whose nucleons are in a configuration that does not permit them the surplus energy required to produce a radioactive emission. The nuclei of such isotopes are not radioactive and unlike radionuclides do not spontaneously undergo radioactive decay. When these nuclides are referred to in relation to specific elements they are usually called that element's stable isotopes. The 80 elements with one or more stable isotopes comprise a total of 251 nuclides that have not been shown to decay using current equipment. Of these 80 elements, 26 have only one stable isotope and are called monoisotopic. The other 56 have more than one stable isotope. Tin has ten stable isotopes, the largest number of any element. Definition of stability, and naturally occurring nuclides Most naturally occurring nuclides are stable (about 251; see list at the end of this article), and about 35 more (total of 286) are known to be radioactive with long eno ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stable Isotope
Stable nuclides are Isotope, isotopes of a chemical element whose Nucleon, nucleons are in a configuration that does not permit them the surplus energy required to produce a radioactive emission. The Atomic nucleus, nuclei of such isotopes are not radioactive and unlike radionuclides do not spontaneously undergo radioactive decay. When these nuclides are referred to in relation to specific elements they are usually called that element's stable isotopes. The 80 elements with one or more stable isotopes comprise a total of 251 nuclides that have not been shown to decay using current equipment. Of these 80 elements, 26 have only one stable isotope and are called monoisotopic element, monoisotopic. The other 56 have more than one stable isotope. Tin has ten stable isotopes, the largest number of any element. Definition of stability, and naturally occurring nuclides Most naturally occurring nuclides are stable (about 251; see list at the end of this article), and about 35 more (tot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron-56
Iron-56 (56Fe) is the most common isotope of iron. About 91.754% of all iron is iron-56. Of all nuclides, iron-56 has the lowest mass per nucleon. With 8.8 MeV binding energy per nucleon, iron-56 is one of the most tightly bound nuclei. The high nuclear binding energy for 56Fe represents the point where further nuclear reactions become energetically unfavorable. Because of this, it is among the heaviest elements formed in stellar nucleosynthesis reactions in massive stars. These reactions fuse lighter elements like magnesium, silicon, and sulfur to form heavier elements. Among the heavier elements formed is 56Ni, which subsequently decays to 56Co and then 56Fe. Relationship to nickel-62 Nickel-62, a relatively rare isotope of nickel, has a higher nuclear binding energy per nucleon; this is consistent with having a higher mass-per-nucleon because nickel-62 has a greater proportion of neutrons, which are slightly more massive than protons. (See the nickel-62 article ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |