silicon-based life form on:
[Wikipedia]
[Google]
[Amazon]
 Several forms of
Several forms of
 A shadow biosphere is a hypothetical microbial
A shadow biosphere is a hypothetical microbial


 The silicon atom has been much discussed as the basis for an alternative biochemical system, because silicon has many chemical similarities to carbon and is in the same group of the periodic table. Like carbon, silicon can create molecules that are sufficiently large to carry biological information.
However, silicon has several drawbacks as a carbon alternative. Carbon is ten times more cosmically abundant than silicon, and its chemistry appears naturally more complex. By 1998, astronomers had identified 84 carbon-containing molecules in the
The silicon atom has been much discussed as the basis for an alternative biochemical system, because silicon has many chemical similarities to carbon and is in the same group of the periodic table. Like carbon, silicon can create molecules that are sufficiently large to carry biological information.
However, silicon has several drawbacks as a carbon alternative. Carbon is ten times more cosmically abundant than silicon, and its chemistry appears naturally more complex. By 1998, astronomers had identified 84 carbon-containing molecules in the
The Limits of Organic Life in Planetary Systems
The National Academies Press, 2007; pages 69–79. Solvents discussed by the Baross committee include
The Limits of Organic Life in Planetary Systems
The National Academies Press, 2007; p. 72.
The Limits of Organic Life in Planetary Systems
The National Academies Press, 2007; p. 73. formamide,Committee on the Limits of Organic Life in Planetary Systems, Committee on the Origins and Evolution of Life, National Research Council
The Limits of Organic Life in Planetary Systems
The National Academies Press, 2007; p. 74. hydrocarbons, and (at temperatures much lower than Earth's) liquid
The Limits of Organic Life in Planetary Systems
The National Academies Press, 2007; p. 75. Water as a solvent limits the forms biochemistry can take. For example, Steven Benner, proposes the polyelectrolyte theory of the gene that claims that for a genetic
The Limits of Organic Life in Planetary Systems
The National Academies Press, 2007; page 70. For instance, water ice has a high
 A
A
The Limits of Organic Life in Planetary Systems
The National Academies Press, 2007; page 74. Water is a stronger solvent than the hydrocarbons, enabling easier transport of substances in a cell. However, water is also more chemically reactive and can break down large organic molecules through hydrolysis. A life-form whose solvent was a hydrocarbon would not face the threat of its biomolecules being destroyed in this way. Also, the water molecule's tendency to form strong hydrogen bonds can interfere with internal hydrogen bonding in complex organic molecules. Life with a hydrocarbon solvent could make more use of hydrogen bonds within its biomolecules. Moreover, the strength of hydrogen bonds within biomolecules would be appropriate to a low-temperature biochemistry. Astrobiologist Chris McKay has argued, on thermodynamic grounds, that if life does exist on Titan's surface, using hydrocarbons as a solvent, it is likely also to use the more complex hydrocarbons as an energy source by reacting them with hydrogen, reducing ethane and
An analysis of data obtained using the Atacama Large Millimeter / submillimeter Array (ALMA), completed in 2017, confirmed substantial amounts of acrylonitrile in Titan's atmosphere. Later studies questioned whether acrylonitrile would be able to self-assemble into azotosomes.
 Other solvents sometimes proposed:
* Supercritical fluids: supercritical carbon dioxide and supercritical hydrogen.
* Simple hydrogen compounds:
Other solvents sometimes proposed:
* Supercritical fluids: supercritical carbon dioxide and supercritical hydrogen.
* Simple hydrogen compounds:
 The
The
The Limits of Organic Life in Planetary Systems
The National Academies Press, 2007.Committee on the Limits of Organic Life in Planetary Systems, Committee on the Origins and Evolution of Life, National Research Council
The Limits of Organic Life in Planetary Systems
The National Academies Press, 2007; page 5
Astronomy FAQ
{{Portal bar, Astronomy, Biology, Space Astrobiology Science fiction themes Biological hypotheses Scientific speculation
 Several forms of
Several forms of biochemistry
Biochemistry, or biological chemistry, is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology, a ...
are agreed to be scientifically viable but are not proven to exist at this time. The kinds of living organisms currently known on Earth all use carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
compounds for basic structural and metabolic
Metabolism (, from ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run cellular processes; the ...
functions, water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known liv ...
as a solvent
A solvent (from the Latin language, Latin ''wikt:solvo#Latin, solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a Solution (chemistry), solution. A solvent is usually a liquid but can also be a solid, a gas ...
, and DNA
Deoxyribonucleic acid (; DNA) is a polymer composed of two polynucleotide chains that coil around each other to form a double helix. The polymer carries genetic instructions for the development, functioning, growth and reproduction of al ...
or RNA
Ribonucleic acid (RNA) is a polymeric molecule that is essential for most biological functions, either by performing the function itself (non-coding RNA) or by forming a template for the production of proteins (messenger RNA). RNA and deoxyrib ...
to define and control their form. If life
Life, also known as biota, refers to matter that has biological processes, such as Cell signaling, signaling and self-sustaining processes. It is defined descriptively by the capacity for homeostasis, Structure#Biological, organisation, met ...
exists on other planet
A planet is a large, Hydrostatic equilibrium, rounded Astronomical object, astronomical body that is generally required to be in orbit around a star, stellar remnant, or brown dwarf, and is not one itself. The Solar System has eight planets b ...
s or moons it may be chemically similar, though it is also possible that there are organisms with quite different chemistries for instance, involving other classes of carbon compounds, compounds of another element, or another solvent in place of water.
The possibility of life-forms being based on "alternative" biochemistries is the topic of an ongoing scientific discussion, informed by what is known about extraterrestrial environments and about the chemical behaviour of various elements and compounds. It is of interest in synthetic biology
Synthetic biology (SynBio) is a multidisciplinary field of science that focuses on living systems and organisms. It applies engineering principles to develop new biological parts, devices, and systems or to redesign existing systems found in nat ...
and is also a common subject in science fiction.
The element silicon
Silicon is a chemical element; it has symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid (sometimes considered a non-metal) and semiconductor. It is a membe ...
has been much discussed as a hypothetical alternative to carbon. Silicon is in the same group as carbon on the periodic table
The periodic table, also known as the periodic table of the elements, is an ordered arrangement of the chemical elements into rows (" periods") and columns (" groups"). It is an icon of chemistry and is widely used in physics and other s ...
and, like carbon, it is tetravalent. Hypothetical alternatives to water include ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
, which, like water, is a polar molecule, and cosmically abundant; and non-polar hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and Hydrophobe, hydrophobic; their odor is usually fain ...
solvents such as methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes ...
and ethane
Ethane ( , ) is a naturally occurring Organic compound, organic chemical compound with chemical formula . At standard temperature and pressure, ethane is a colorless, odorless gas. Like many hydrocarbons, ethane is List of purification methods ...
, which are known to exist in liquid form on the surface of Titan.
Overview of hypothetical types of biochemistry
Shadow biosphere
biosphere
The biosphere (), also called the ecosphere (), is the worldwide sum of all ecosystems. It can also be termed the zone of life on the Earth. The biosphere (which is technically a spherical shell) is virtually a closed system with regard to mat ...
of Earth that uses radically different biochemical
Biochemistry, or biological chemistry, is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology, ...
and molecular processes than currently known life. Although life on Earth is relatively well-studied, the shadow biosphere may still remain unnoticed because the exploration of the microbial world targets primarily the biochemistry of the macro-organisms.
Alternative-chirality biomolecules
Perhaps the least unusual alternative biochemistry would be one with differing chirality of its biomolecules. In known Earth-based life,amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 a ...
s are almost universally of the form and sugar
Sugar is the generic name for sweet-tasting, soluble carbohydrates, many of which are used in food. Simple sugars, also called monosaccharides, include glucose
Glucose is a sugar with the Chemical formula#Molecular formula, molecul ...
s are of the form. Molecules using amino acids or sugars may be possible; molecules of such a chirality, however, would be incompatible with organisms using the opposing chirality molecules. Amino acids whose chirality is opposite to the norm are found on Earth, and these substances are generally thought to result from decay of organisms of normal chirality. However, physicist Paul Davies speculates that some of them might be products of "anti-chiral" life.
It is questionable, however, whether such a biochemistry would be truly alien. Although it would certainly be an alternative stereochemistry
Stereochemistry, a subdiscipline of chemistry, studies the spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereoisomers, which are defined ...
, molecules that are overwhelmingly found in one enantiomer
In chemistry, an enantiomer (Help:IPA/English, /ɪˈnænti.əmər, ɛ-, -oʊ-/ Help:Pronunciation respelling key, ''ih-NAN-tee-ə-mər''), also known as an optical isomer, antipode, or optical antipode, is one of a pair of molecular entities whi ...
throughout the vast majority of organisms can nonetheless often be found in another enantiomer in different (often basal) organisms such as in comparisons between members of Archaea
Archaea ( ) is a Domain (biology), domain of organisms. Traditionally, Archaea only included its Prokaryote, prokaryotic members, but this has since been found to be paraphyletic, as eukaryotes are known to have evolved from archaea. Even thou ...
and other domains, making it an open topic whether an alternative stereochemistry is truly novel.
Non-carbon-based biochemistries
On Earth, all known living things have a carbon-based structure and system. Scientists have speculated about the advantages and disadvantages of using elements other than carbon to form the molecular structures necessary for life, but no one has proposed a theory employing such atoms to form all the necessary structures. However, as Carl Sagan argued, it is very difficult to be certain whether a statement that applies to all life on Earth will turn out to apply to all life throughout the universe. Sagan used the term " carbon chauvinism" for such an assumption. He regardedsilicon
Silicon is a chemical element; it has symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid (sometimes considered a non-metal) and semiconductor. It is a membe ...
and germanium
Germanium is a chemical element; it has Symbol (chemistry), symbol Ge and atomic number 32. It is lustrous, hard-brittle, grayish-white and similar in appearance to silicon. It is a metalloid or a nonmetal in the carbon group that is chemically ...
as conceivable alternatives to carbon (other plausible elements include but are not limited to palladium and titanium
Titanium is a chemical element; it has symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resistant to corrosion in ...
); but, on the other hand, he noted that carbon does seem more chemically versatile and is more abundant in the cosmos. Norman Horowitz devised the experiments to determine whether life might exist on Mars that were carried out by the Viking Lander of 1976, the first U.S. mission to successfully land a probe on the surface of Mars. Horowitz argued that the great versatility of the carbon atom makes it the element most likely to provide solutions, even exotic solutions, to the problems of survival on other planets.Horowitz, N.H. (1986). Utopia and Back and the search for life in the solar system. New York: W.H. Freeman and Company. . He considered that there was only a remote possibility that non-carbon life forms could exist with genetic information systems capable of self-replication and the ability to evolve and adapt.
Silicon biochemistry

 The silicon atom has been much discussed as the basis for an alternative biochemical system, because silicon has many chemical similarities to carbon and is in the same group of the periodic table. Like carbon, silicon can create molecules that are sufficiently large to carry biological information.
However, silicon has several drawbacks as a carbon alternative. Carbon is ten times more cosmically abundant than silicon, and its chemistry appears naturally more complex. By 1998, astronomers had identified 84 carbon-containing molecules in the
The silicon atom has been much discussed as the basis for an alternative biochemical system, because silicon has many chemical similarities to carbon and is in the same group of the periodic table. Like carbon, silicon can create molecules that are sufficiently large to carry biological information.
However, silicon has several drawbacks as a carbon alternative. Carbon is ten times more cosmically abundant than silicon, and its chemistry appears naturally more complex. By 1998, astronomers had identified 84 carbon-containing molecules in the interstellar medium
The interstellar medium (ISM) is the matter and radiation that exists in the outer space, space between the star systems in a galaxy. This matter includes gas in ionic, atomic, and molecular form, as well as cosmic dust, dust and cosmic rays. It f ...
, but only 8 containing silicon, of which half also included carbon. Even though Earth
Earth is the third planet from the Sun and the only astronomical object known to Planetary habitability, harbor life. This is enabled by Earth being an ocean world, the only one in the Solar System sustaining liquid surface water. Almost all ...
and other terrestrial planet
A terrestrial planet, tellurian planet, telluric planet, or rocky planet, is a planet that is composed primarily of silicate, rocks or metals. Within the Solar System, the terrestrial planets accepted by the IAU are the inner planets closest to ...
s are exceptionally silicon-rich and carbon-poor (silicon is roughly 925 times more abundant in Earth's crust than carbon), terrestrial life bases itself on carbon. It may avoid silicon because silicon compounds are less varied, unstable in the presence of water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known liv ...
, or block the flow of heat.
Relative to carbon, silicon has a much larger atomic radius, and forms much weaker covalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
s to atoms — except oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
and fluorine
Fluorine is a chemical element; it has Chemical symbol, symbol F and atomic number 9. It is the lightest halogen and exists at Standard temperature and pressure, standard conditions as pale yellow Diatomic molecule, diatomic gas. Fluorine is extre ...
, with which it forms very strong bonds. Almost no multiple bond
Multiple may refer to:
Economics
*Multiple finance, a method used to analyze stock prices
*Multiples of the price-to-earnings ratio
*Chain stores, are also referred to as 'Multiples'
*Box office multiple, the ratio of a film's total gross to tha ...
s to silicon are stable, although silicon does exhibit varied coordination number
In chemistry, crystallography, and materials science, the coordination number, also called ligancy, of a central atom in a molecule or crystal is the number of atoms, molecules or ions bonded to it. The ion/molecule/atom surrounding the central ion ...
. Silanes, silicon analogues to the alkanes, react rapidly with water, and long-chain silanes spontaneously decompose. Consequently, most terrestrial silicon is "locked up" in silica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundant f ...
, and not a wide variety of biogenic precursors.
Silicone
In Organosilicon chemistry, organosilicon and polymer chemistry, a silicone or polysiloxane is a polymer composed of repeating units of siloxane (, where R = Organyl group, organic group). They are typically colorless oils or elastomer, rubber ...
s, which alternate between silicon and oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
atoms, are much more stable than silanes, and may even be more stable than the equivalent hydrocarbons in sulfuric acid-rich extraterrestrial environments. Alternatively, the weak bonds in silicon compounds may help maintain a rapid pace of life at cryogenic
In physics, cryogenics is the production and behaviour of materials at very low temperatures.
The 13th International Institute of Refrigeration's (IIR) International Congress of Refrigeration (held in Washington, DC in 1971) endorsed a univers ...
temperatures. Polysilanols, the silicon homologues to sugar
Sugar is the generic name for sweet-tasting, soluble carbohydrates, many of which are used in food. Simple sugars, also called monosaccharides, include glucose
Glucose is a sugar with the Chemical formula#Molecular formula, molecul ...
s, are among the few compounds soluble in liquid nitrogen
Liquid nitrogen (LN2) is nitrogen in a liquid state at cryogenics, low temperature. Liquid nitrogen has a boiling point of about . It is produced industrially by fractional distillation of liquid air. It is a colorless, mobile liquid whose vis ...
.
All known silicon macromolecules are artificial polymers, and so "monotonous compared with the combinatorial universe of organic macromolecules". Even so, some Earth life uses biogenic silica: diatom
A diatom (Neo-Latin ''diatoma'') is any member of a large group comprising several Genus, genera of algae, specifically microalgae, found in the oceans, waterways and soils of the world. Living diatoms make up a significant portion of Earth's B ...
s' silicate skeleton
A skeleton is the structural frame that supports the body of most animals. There are several types of skeletons, including the exoskeleton, which is a rigid outer shell that holds up an organism's shape; the endoskeleton, a rigid internal fra ...
s. A. G. Cairns-Smith hypothesized that silicate minerals in water played a crucial role in abiogenesis, in that biogenic carbon compounds formed around their crystal structures. Although not observed in nature, carbon–silicon bonds have been added to biochemistry under directed evolution (artificial selection): a cytochrome ''c'' protein from '' Rhodothermus marinus'' has been engineered to catalyze new carbon–silicon bonds between hydrosilanes and diazo compounds.
Other exotic element-based biochemistries
* Boranes are dangerously explosive in Earth's atmosphere, but would be more stable in a reducing atmosphere. However, boron's low cosmic abundance makes it less likely as a base for life than carbon. * Various metals, together with oxygen, can form very complex and thermally stable structures rivaling those of organic compounds; the heteropoly acids are one such family. Some metal oxides are also similar to carbon in their ability to form both nanotube structures and diamond-like crystals (such as cubic zirconia).Titanium
Titanium is a chemical element; it has symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resistant to corrosion in ...
, aluminium
Aluminium (or aluminum in North American English) is a chemical element; it has chemical symbol, symbol Al and atomic number 13. It has a density lower than that of other common metals, about one-third that of steel. Aluminium has ...
, magnesium
Magnesium is a chemical element; it has Symbol (chemistry), symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 ...
, and iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
are all more abundant in the Earth's crust than carbon. Metal-oxide-based life could therefore be a possibility under certain conditions, including those (such as high temperatures) at which carbon-based life would be unlikely. The Cronin group at Glasgow University reported self-assembly of tungsten polyoxometalates into cell-like spheres. By modifying their metal oxide content, the spheres can acquire holes that act as porous membrane, selectively allowing chemicals in and out of the sphere according to size.
* Sulfur
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms ...
is also able to form long-chain molecules, but suffers from the same high-reactivity problems as phosphorus and silanes. The biological use of sulfur as an alternative to carbon is purely hypothetical, especially because sulfur usually forms only linear chains rather than branched ones. (The biological use of sulfur as an electron acceptor is widespread and can be traced back 3.5 billion years on Earth, thus predating the use of molecular oxygen. Sulfur-reducing bacteria can utilize elemental sulfur instead of oxygen, reducing sulfur to hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is toxic, corrosive, and flammable. Trace amounts in ambient atmosphere have a characteristic foul odor of rotten eggs. Swedish chemist ...
.)
Arsenic as an alternative to phosphorus
Whilearsenic
Arsenic is a chemical element; it has Symbol (chemistry), symbol As and atomic number 33. It is a metalloid and one of the pnictogens, and therefore shares many properties with its group 15 neighbors phosphorus and antimony. Arsenic is not ...
, which is chemically similar to phosphorus
Phosphorus is a chemical element; it has Chemical symbol, symbol P and atomic number 15. All elemental forms of phosphorus are highly Reactivity (chemistry), reactive and are therefore never found in nature. They can nevertheless be prepared ar ...
, is poisonous for most life forms on Earth, it is incorporated into the biochemistry of some organisms. Some marine algae incorporate arsenic into complex organic molecules such as arsenosugars and arsenobetaines. Fungi
A fungus (: fungi , , , or ; or funguses) is any member of the group of eukaryotic organisms that includes microorganisms such as yeasts and mold (fungus), molds, as well as the more familiar mushrooms. These organisms are classified as one ...
and bacteria
Bacteria (; : bacterium) are ubiquitous, mostly free-living organisms often consisting of one Cell (biology), biological cell. They constitute a large domain (biology), domain of Prokaryote, prokaryotic microorganisms. Typically a few micr ...
can produce volatile methylated arsenic compounds. Arsenate reduction and arsenite oxidation have been observed in microbes
A microorganism, or microbe, is an organism of microscopic size, which may exist in its single-celled form or as a colony of cells. The possible existence of unseen microbial life was suspected from antiquity, with an early attestation in ...
('' Chrysiogenes arsenatis''). Additionally, some prokaryote
A prokaryote (; less commonly spelled procaryote) is a unicellular organism, single-celled organism whose cell (biology), cell lacks a cell nucleus, nucleus and other membrane-bound organelles. The word ''prokaryote'' comes from the Ancient Gree ...
s can use arsenate as a terminal electron acceptor during anaerobic growth and some can utilize arsenite as an electron donor to generate energy.
It has been speculated that the earliest life forms on Earth may have used arsenic biochemistry in place of phosphorus in the structure of their DNA. A common objection to this scenario is that arsenate esters are so much less stable to hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
than corresponding phosphate esters
Phosphates are the naturally occurring form of the element phosphorus.
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphor ...
that arsenic is poorly suited for this function.
The authors of a 2010 geomicrobiology study, supported in part by NASA, have postulated that a bacterium, named GFAJ-1, collected in the sediments of Mono Lake
Mono Lake ( ) is a Salt lake, saline soda lake in Mono County, California, formed at least 760,000 years ago as a terminal lake in an endorheic basin. The lack of an outlet causes Hypersaline lake, high levels of salts to accumulate in the lake ...
in eastern California
California () is a U.S. state, state in the Western United States that lies on the West Coast of the United States, Pacific Coast. It borders Oregon to the north, Nevada and Arizona to the east, and shares Mexico–United States border, an ...
, can employ such 'arsenic DNA' when cultured without phosphorus. They proposed that the bacterium may employ high levels of poly-β-hydroxybutyrate or other means to reduce the effective concentration of water and stabilize its arsenate esters. This claim was heavily criticized almost immediately after publication for the perceived lack of appropriate controls. Science writer Carl Zimmer contacted several scientists for an assessment: "I reached out to a dozen experts ... Almost unanimously, they think the NASA scientists have failed to make their case".
Other authors were unable to reproduce their results and showed that the study had issues with phosphate contamination, suggesting that the low amounts present could sustain extremophile lifeforms.
Alternatively, it was suggested that GFAJ-1 cells grow by recycling phosphate from degraded ribosomes, rather than by replacing it with arsenate.
Non-water solvents
In addition to carbon compounds, all currently known terrestrial life also requires water as a solvent. This has led to discussions about whether water is the only liquid capable of filling that role. The idea that an extraterrestrial life-form might be based on a solvent other than water has been taken seriously in recent scientific literature by the biochemist Steven Benner, and by the astrobiological committee chaired by John A. Baross.Committee on the Limits of Organic Life in Planetary Systems, Committee on the Origins and Evolution of Life, National Research CouncilThe Limits of Organic Life in Planetary Systems
The National Academies Press, 2007; pages 69–79. Solvents discussed by the Baross committee include
ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
,Committee on the Limits of Organic Life in Planetary Systems, Committee on the Origins and Evolution of Life, National Research CouncilThe Limits of Organic Life in Planetary Systems
The National Academies Press, 2007; p. 72.
sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
,Committee on the Limits of Organic Life in Planetary Systems, Committee on the Origins and Evolution of Life, National Research CouncilThe Limits of Organic Life in Planetary Systems
The National Academies Press, 2007; p. 73. formamide,Committee on the Limits of Organic Life in Planetary Systems, Committee on the Origins and Evolution of Life, National Research Council
The Limits of Organic Life in Planetary Systems
The National Academies Press, 2007; p. 74. hydrocarbons, and (at temperatures much lower than Earth's) liquid
nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
, or hydrogen in the form of a supercritical fluid.Committee on the Limits of Organic Life in Planetary Systems, Committee on the Origins and Evolution of Life, National Research CouncilThe Limits of Organic Life in Planetary Systems
The National Academies Press, 2007; p. 75. Water as a solvent limits the forms biochemistry can take. For example, Steven Benner, proposes the polyelectrolyte theory of the gene that claims that for a genetic
biopolymer
Biopolymers are natural polymers produced by the cells of living organisms. Like other polymers, biopolymers consist of monomeric units that are covalently bonded in chains to form larger molecules. There are three main classes of biopolymers, ...
such as DNA to function in water, it requires repeated ionic charges. If water is not required for life, these limits on genetic biopolymers are removed.
Carl Sagan once described himself as both a carbon chauvinist and a water chauvinist; however, on another occasion he said that he was a carbon chauvinist but "not that much of a water chauvinist".
He speculated on hydrocarbons, hydrofluoric acid, and ammonia as possible alternatives to water.
Some of the properties of water that are important for life processes include:
* A complexity which leads to a large number of permutations of possible reaction paths including acid–base chemistry, H+ cations, OH− anions, hydrogen bonding, van der Waals bonding, dipole–dipole and other polar interactions, aqueous solvent cages, and hydrolysis. This complexity offers a large number of pathways for evolution to produce life, many other solvents have dramatically fewer possible reactions, which severely limits evolution.
* Thermodynamic stability: the free energy of formation of liquid water is low enough (−237.24 kJ/mol) that water undergoes few reactions. Other solvents are highly reactive, particularly with oxygen.
* Water does not combust in oxygen because it is already the combustion product of hydrogen with oxygen. Most alternative solvents are not stable in an oxygen-rich atmosphere, so it is highly unlikely that those liquids could support aerobic life.
* A large temperature range over which it is liquid
Liquid is a state of matter with a definite volume but no fixed shape. Liquids adapt to the shape of their container and are nearly incompressible, maintaining their volume even under pressure. The density of a liquid is usually close to th ...
.
* High solubility of oxygen and carbon dioxide at room temperature supporting the evolution of aerobic aquatic plant and animal life.
* A high heat capacity (leading to higher environmental temperature stability).
* Water is a room-temperature liquid leading to a large population of quantum transition states required to overcome reaction barriers. Cryogenic liquids (such as liquid methane) have exponentially lower transition state populations which are needed for life based on chemical reactions. This leads to chemical reaction rates which may be so slow as to preclude the development of any life based on chemical reactions.
* Spectroscopic transparency allowing solar radiation to penetrate several meters into the liquid (or solid), greatly aiding the evolution of aquatic life.
* A large heat of vaporization leading to stable lakes and oceans.
* The ability to dissolve a wide variety of compounds.
* The solid (ice) has lower density than the liquid, so ice floats on the liquid. This is why bodies of water freeze over but do not freeze solid (from the bottom up). If ice were denser than liquid water (as is true for nearly all other compounds), then large bodies of liquid would slowly freeze solid, which would not be conducive to the formation of life.
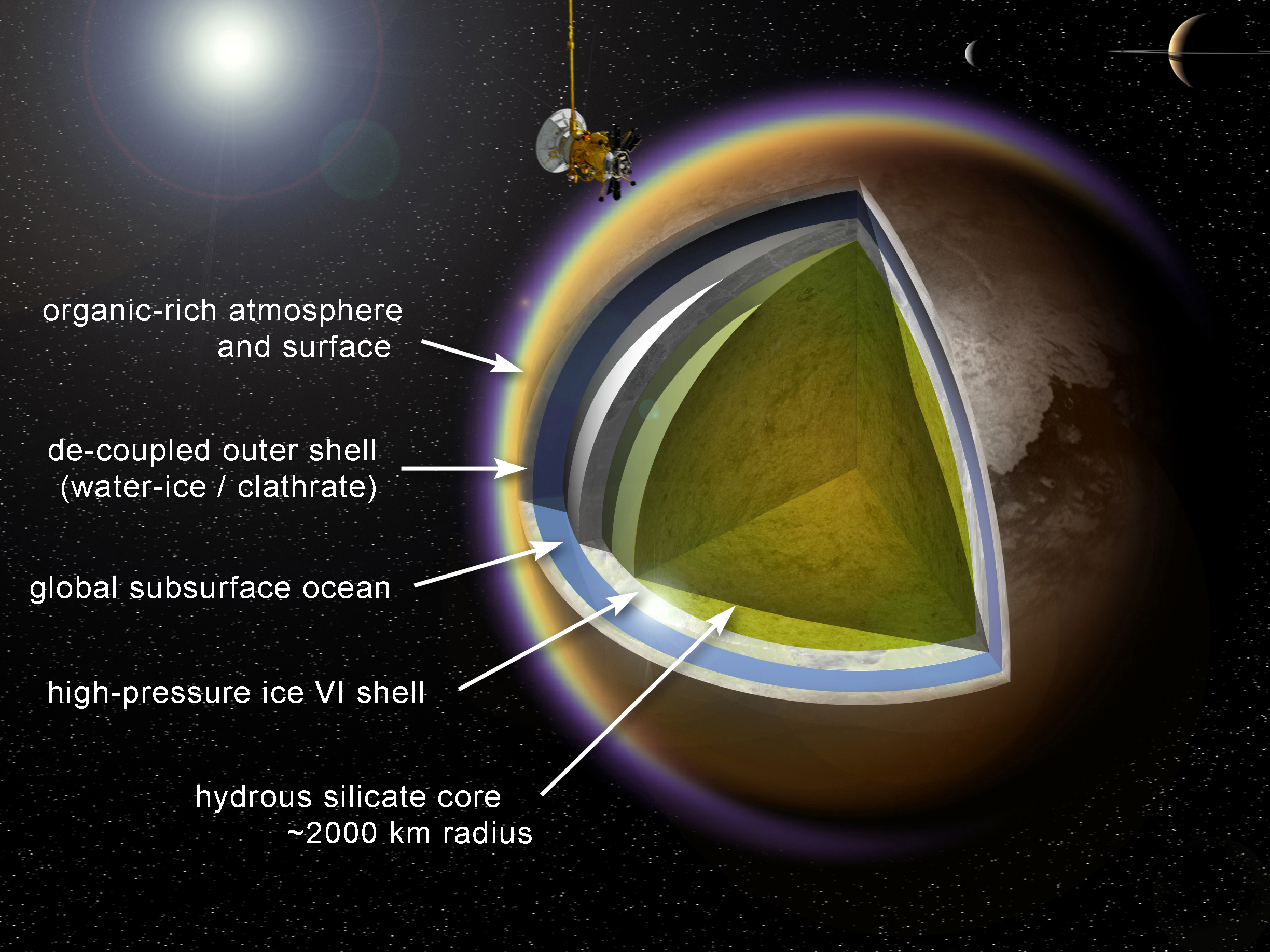
Water as a compound is cosmically abundant, although much of it is in the form of vapor or ice. Subsurface liquid water is considered likely or possible on several of the outer moons: Enceladus (where geysers have been observed), Europa, Titan, and Ganymede. Earth and Titan are the only worlds currently known to have stable bodies of liquid on their surfaces.
Not all properties of water are necessarily advantageous for life, however.Committee on the Limits of Organic Life in Planetary Systems, Committee on the Origins and Evolution of Life, National Research CouncilThe Limits of Organic Life in Planetary Systems
The National Academies Press, 2007; page 70. For instance, water ice has a high
albedo
Albedo ( ; ) is the fraction of sunlight that is Diffuse reflection, diffusely reflected by a body. It is measured on a scale from 0 (corresponding to a black body that absorbs all incident radiation) to 1 (corresponding to a body that reflects ...
, meaning that it reflects a significant quantity of light and heat from the Sun. During ice age
An ice age is a long period of reduction in the temperature of Earth's surface and atmosphere, resulting in the presence or expansion of continental and polar ice sheets and alpine glaciers. Earth's climate alternates between ice ages, and g ...
s, as reflective ice builds up over the surface of the water, the effects of global cooling are increased.
There are some properties that make certain compounds and elements much more favorable than others as solvents in a successful biosphere. The solvent must be able to exist in liquid equilibrium over a range of temperatures the planetary object would normally encounter. Because boiling points vary with the pressure, the question tends not to be ''does'' the prospective solvent remain liquid, but ''at what pressure''. For example, hydrogen cyanide
Hydrogen cyanide (formerly known as prussic acid) is a chemical compound with the chemical formula, formula HCN and structural formula . It is a highly toxic and flammable liquid that boiling, boils slightly above room temperature, at . HCN is ...
has a narrow liquid-phase temperature range at 1 atmosphere, but in an atmosphere with the pressure of Venus
Venus is the second planet from the Sun. It is often called Earth's "twin" or "sister" planet for having almost the same size and mass, and the closest orbit to Earth's. While both are rocky planets, Venus has an atmosphere much thicker ...
, with of pressure, it can indeed exist in liquid form over a wide temperature range.
Ammonia
Theammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
molecule (NH3), like the water molecule, is abundant in the universe, being a compound of hydrogen (the simplest and most common element) with another very common element, nitrogen. The possible role of liquid ammonia as an alternative solvent for life is an idea that goes back at least to 1954, when J. B. S. Haldane raised the topic at a symposium about life's origin. cited in
Numerous chemical reactions are possible in an ammonia solution, and liquid ammonia has chemical similarities with water. Ammonia can dissolve most organic molecules at least as well as water does and, in addition, it is capable of dissolving many elemental metals. Haldane made the point that various common water-related organic compounds have ammonia-related analogs; for instance the ammonia-related amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
group (−NH2) is analogous to the water-related hydroxyl group (−OH).
Ammonia, like water, can either accept or donate an H+ ion. When ammonia accepts an H+, it forms the ammonium
Ammonium is a modified form of ammonia that has an extra hydrogen atom. It is a positively charged (cationic) polyatomic ion, molecular ion with the chemical formula or . It is formed by the protonation, addition of a proton (a hydrogen nucleu ...
cation (NH4+), analogous to hydronium (H3O+). When it donates an H+ ion, it forms the amide anion (NH2−), analogous to the hydroxide anion (OH−). Compared to water, however, ammonia is more inclined to accept an H+ ion, and less inclined to donate one; it is a stronger nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
. Ammonia added to water functions as an Arrhenius base: it increases the concentration of the anion hydroxide. Conversely, using a solvent system definition of acidity and basicity, water added to liquid ammonia functions as an acid, because it increases the concentration of the cation ammonium. The carbonyl group (C=O), which is much used in terrestrial biochemistry, would not be stable in ammonia solution, but the analogous imine group (C=NH) could be used instead.
However, ammonia has some problems as a basis for life. The hydrogen bonds between ammonia molecules are weaker than those in water, causing ammonia's heat of vaporization to be half that of water, its surface tension
Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. Surface tension (physics), tension is what allows objects with a higher density than water such as razor blades and insects (e.g. Ge ...
to be a third, and reducing its ability to concentrate non-polar molecules through a hydrophobic
In chemistry, hydrophobicity is the chemical property of a molecule (called a hydrophobe) that is seemingly repelled from a mass of water. In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to be nonpolar and, thu ...
effect. Gerald Feinberg and Robert Shapiro have questioned whether ammonia could hold prebiotic molecules together well enough to allow the emergence of a self-reproducing system. Ammonia is also flammable in oxygen and could not exist sustainably in an environment suitable for aerobic metabolism.
 A
A biosphere
The biosphere (), also called the ecosphere (), is the worldwide sum of all ecosystems. It can also be termed the zone of life on the Earth. The biosphere (which is technically a spherical shell) is virtually a closed system with regard to mat ...
based on ammonia would likely exist at temperatures or air pressures that are extremely unusual in relation to life on Earth. Life on Earth usually exists between the melting point and boiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding envi ...
of water, at a pressure designated as normal pressure, between . When also held to normal pressure, ammonia's melting and boiling points are and respectively. Because chemical reactions generally proceed more slowly at lower temperatures, ammonia-based life existing in this set of conditions might metabolize more slowly and evolve more slowly than life on Earth. On the other hand, lower temperatures could also enable living systems to use chemical species that would be too unstable at Earth temperatures to be useful.
A set of conditions where ammonia is liquid at Earth-like temperatures would involve it being at a much higher pressure. For example, at 60 atm ammonia melts at and boils at .
Ammonia and ammonia–water mixtures remain liquid at temperatures far below the freezing point of pure water, so such biochemistries might be well suited to planets and moons orbiting outside the water-based habitability zone. Such conditions could exist, for example, under the surface of Saturn
Saturn is the sixth planet from the Sun and the second largest in the Solar System, after Jupiter. It is a gas giant, with an average radius of about 9 times that of Earth. It has an eighth the average density of Earth, but is over 95 tim ...
's largest moon Titan.
Methane and other hydrocarbons
Methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes ...
(CH4) is a simple hydrocarbon: that is, a compound of two of the most common elements in the cosmos: hydrogen and carbon. It has a cosmic abundance comparable with ammonia. Hydrocarbons could act as a solvent over a wide range of temperatures, but would lack polarity. Isaac Asimov, the biochemist
Biochemists are scientists who are trained in biochemistry. They study chemical processes and chemical transformations in living organisms. Biochemists study DNA, proteins and Cell (biology), cell parts. The word "biochemist" is a portmanteau of ...
and science fiction writer, suggested in 1981 that poly-lipids
Lipids are a broad group of organic compounds which include fats, waxes, sterols, fat-soluble vitamins (such as vitamins Vitamin A, A, Vitamin D, D, Vitamin E, E and Vitamin K, K), monoglycerides, diglycerides, phospholipids, and others. The fu ...
could form a substitute for proteins in a non-polar solvent such as methane. Lakes composed of a mixture of hydrocarbons, including methane and ethane
Ethane ( , ) is a naturally occurring Organic compound, organic chemical compound with chemical formula . At standard temperature and pressure, ethane is a colorless, odorless gas. Like many hydrocarbons, ethane is List of purification methods ...
, have been detected on the surface of Titan by the ''Cassini'' spacecraft.
There is debate about the effectiveness of methane and other hydrocarbons as a solvent for life compared to water or ammonia.Committee on the Limits of Organic Life in Planetary Systems, Committee on the Origins and Evolution of Life, National Research CouncilThe Limits of Organic Life in Planetary Systems
The National Academies Press, 2007; page 74. Water is a stronger solvent than the hydrocarbons, enabling easier transport of substances in a cell. However, water is also more chemically reactive and can break down large organic molecules through hydrolysis. A life-form whose solvent was a hydrocarbon would not face the threat of its biomolecules being destroyed in this way. Also, the water molecule's tendency to form strong hydrogen bonds can interfere with internal hydrogen bonding in complex organic molecules. Life with a hydrocarbon solvent could make more use of hydrogen bonds within its biomolecules. Moreover, the strength of hydrogen bonds within biomolecules would be appropriate to a low-temperature biochemistry. Astrobiologist Chris McKay has argued, on thermodynamic grounds, that if life does exist on Titan's surface, using hydrocarbons as a solvent, it is likely also to use the more complex hydrocarbons as an energy source by reacting them with hydrogen, reducing ethane and
acetylene
Acetylene (Chemical nomenclature, systematic name: ethyne) is a chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is u ...
to methane. Possible evidence for this form of life on Titan was identified in 2010 by Darrell Strobel of Johns Hopkins University
The Johns Hopkins University (often abbreviated as Johns Hopkins, Hopkins, or JHU) is a private university, private research university in Baltimore, Maryland, United States. Founded in 1876 based on the European research institution model, J ...
; a greater abundance of molecular hydrogen in the upper atmospheric layers of Titan compared to the lower layers, arguing for a downward diffusion at a rate of roughly 1025 molecules per second and disappearance of hydrogen near Titan's surface. As Strobel noted, his findings were in line with the effects Chris McKay had predicted if methanogenic life-forms were present. The same year, another study showed low levels of acetylene on Titan's surface, which were interpreted by Chris McKay as consistent with the hypothesis of organisms reducing acetylene to methane. While restating the biological hypothesis, McKay cautioned that other explanations for the hydrogen and acetylene findings are to be considered more likely: the possibilities of yet unidentified physical or chemical processes (e.g. a non-living surface catalyst enabling acetylene to react with hydrogen), or flaws in the current models of material flow. He noted that even a non-biological catalyst effective at 95 K would in itself be a startling discovery.
Azotosome
A hypotheticalcell membrane
The cell membrane (also known as the plasma membrane or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of a cell from the outside environment (the extr ...
termed an azotosome, capable of functioning in liquid methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes ...
in Titan conditions was computer-modeled in an article published in February 2015. Composed of acrylonitrile, a small molecule containing carbon, hydrogen, and nitrogen, it is predicted to have stability and flexibility in liquid methane comparable to that of a phospholipid bilayer (the type of cell membrane possessed by all life on Earth) in liquid water.Life 'not as we know it' possible on Saturn's moon TitanAn analysis of data obtained using the Atacama Large Millimeter / submillimeter Array (ALMA), completed in 2017, confirmed substantial amounts of acrylonitrile in Titan's atmosphere. Later studies questioned whether acrylonitrile would be able to self-assemble into azotosomes.
Hydrogen fluoride
Hydrogen fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluori ...
(HF), like water, is a polar molecule, and due to its polarity it can dissolve many ionic compounds. At atmospheric pressure
Atmospheric pressure, also known as air pressure or barometric pressure (after the barometer), is the pressure within the atmosphere of Earth. The standard atmosphere (symbol: atm) is a unit of pressure defined as , which is equivalent to 1,013. ...
, its melting point is , and its boiling point is ; the difference between the two is a little more than 100 K. HF also makes hydrogen bonds with its neighbor molecules, as do water and ammonia. It has been considered as a possible solvent for life by scientists such as Peter Sneath cited in and Carl Sagan.
HF is dangerous to the systems of molecules that Earth-life is made of, but certain other organic compounds, such as paraffin wax
Paraffin wax (or petroleum wax) is a soft colorless solid derived from petroleum, coal, or oil shale that consists of a mixture of hydrocarbon molecules containing between 20 and 40 carbon atoms. It is solid at room temperature and melting poi ...
es, are stable with it. Like water and ammonia, liquid hydrogen fluoride supports an acid–base chemistry. Using a solvent system definition of acidity and basicity, nitric acid
Nitric acid is an inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into nitrogen oxide, oxides of nitrogen. Most com ...
functions as a base when it is added to liquid HF.
However, hydrogen fluoride is cosmically rare, unlike water, ammonia, and methane.
Hydrogen sulfide
Hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is toxic, corrosive, and flammable. Trace amounts in ambient atmosphere have a characteristic foul odor of rotten eggs. Swedish chemist ...
is the closest chemical analog to water, but is less polar and is a weaker inorganic solvent. Hydrogen sulfide is quite plentiful on Jupiter's moon Io and may be in liquid form a short distance below the surface; astrobiologist Dirk Schulze-Makuch has suggested it as a possible solvent for life there. On a planet with hydrogen sulfide oceans, the source of the hydrogen sulfide could come from volcanoes, in which case it could be mixed in with a bit of hydrogen fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluori ...
, which could help dissolve minerals. Hydrogen sulfide life might use a mixture of carbon monoxide and carbon dioxide as their carbon source. They might produce and live on sulfur monoxide, which is analogous to oxygen (O2). Hydrogen sulfide, like hydrogen cyanide and ammonia, suffers from the small temperature range where it is liquid, though that, like that of hydrogen cyanide and ammonia, increases with increasing pressure.
Silicon dioxide and silicates
Silicon dioxide
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundan ...
, also known as silica and quartz, is very abundant in the universe and has a large temperature range where it is liquid. However, its melting point is , so it would be impossible to make organic compounds in that temperature, because all of them would decompose. Silicates are similar to silicon dioxide and some have lower melting points than silica. Feinberg and Shapiro have suggested that molten silicate rock could serve as a liquid medium for organisms with a chemistry based on silicon, oxygen, and other elements such as aluminium
Aluminium (or aluminum in North American English) is a chemical element; it has chemical symbol, symbol Al and atomic number 13. It has a density lower than that of other common metals, about one-third that of steel. Aluminium has ...
.
Other solvents or cosolvents
 Other solvents sometimes proposed:
* Supercritical fluids: supercritical carbon dioxide and supercritical hydrogen.
* Simple hydrogen compounds:
Other solvents sometimes proposed:
* Supercritical fluids: supercritical carbon dioxide and supercritical hydrogen.
* Simple hydrogen compounds: hydrogen chloride
The Chemical compound, compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colorless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hyd ...
.
* More complex compounds: sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
, formamide, methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical compound and the simplest aliphatic Alcohol (chemistry), alcohol, with the chemical formula (a methyl group linked to a hydroxyl group, often ab ...
.
* Very-low-temperature fluids: liquid nitrogen
Liquid nitrogen (LN2) is nitrogen in a liquid state at cryogenics, low temperature. Liquid nitrogen has a boiling point of about . It is produced industrially by fractional distillation of liquid air. It is a colorless, mobile liquid whose vis ...
and hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
.
* High-temperature liquids: sodium chloride
Sodium chloride , commonly known as Salt#Edible salt, edible salt, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. It is transparent or translucent, brittle, hygroscopic, and occurs a ...
.
Sulfuric acid in liquid form is strongly polar. It remains liquid at higher temperatures than water, its liquid range being 10 °C to 337 °C at a pressure of 1 atm, although above 300 °C it slowly decomposes. Sulfuric acid is known to be abundant in the clouds of Venus, in the form of aerosol
An aerosol is a suspension (chemistry), suspension of fine solid particles or liquid Drop (liquid), droplets in air or another gas. Aerosols can be generated from natural or Human impact on the environment, human causes. The term ''aerosol'' co ...
droplets. In a biochemistry that used sulfuric acid as a solvent, the alkene group (C=C), with two carbon atoms joined by a double bond, could function analogously to the carbonyl group (C=O) in water-based biochemistry.
A proposal has been made that life on Mars may exist and be using a mixture of water and hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscosity, viscous than Properties of water, water. It is used as an oxidizer, bleaching agent, and antiseptic, usua ...
as its solvent.
A 61.2% (by mass) mix of water and hydrogen peroxide has a freezing point of −56.5 °C and tends to super-cool rather than crystallize. It is also hygroscopic
Hygroscopy is the phenomenon of attracting and holding water molecules via either absorption (chemistry), absorption or adsorption from the surrounding Natural environment, environment, which is usually at normal or room temperature. If water mol ...
, an advantage in a water-scarce environment.
Supercritical carbon dioxide has been proposed as a candidate for alternative biochemistry due to its ability to selectively dissolve organic compounds and assist the functioning of enzymes and because "super-Earth"- or "super-Venus"-type planets with dense high-pressure atmospheres may be common.
Other speculations
Non-green photosynthesizers
Physicists have noted that, although photosynthesis on Earth generally involves green plants, a variety of other-colored plants could also support photosynthesis, essential for most life on Earth, and that other colors might be preferred in places that receive a different mix of stellar radiation than Earth. These studies indicate that blue plants would be unlikely; however yellow or red plants may be relatively common.Variable environments
Many Earth plants and animals undergo major biochemical changes during their life cycles as a response to changing environmental conditions, for example, by having aspore
In biology, a spore is a unit of sexual reproduction, sexual (in fungi) or asexual reproduction that may be adapted for biological dispersal, dispersal and for survival, often for extended periods of time, in unfavourable conditions. Spores fo ...
or hibernation state that can be sustained for years or even millennia between more active life stages. Thus, it would be biochemically possible to sustain life in environments that are only periodically consistent with life as we know it.
For example, frogs in cold climates can survive for extended periods of time with most of their body water in a frozen state, whereas desert frogs in Australia can become inactive and dehydrate in dry periods, losing up to 75% of their fluids, yet return to life by rapidly rehydrating in wet periods. Either type of frog would appear biochemically inactive (i.e. not living) during dormant periods to anyone lacking a sensitive means of detecting low levels of metabolism.
Alanine world and hypothetical alternatives
genetic code
Genetic code is a set of rules used by living cell (biology), cells to Translation (biology), translate information encoded within genetic material (DNA or RNA sequences of nucleotide triplets or codons) into proteins. Translation is accomplished ...
may have evolved during the transition from the RNA world to a protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
world. The Alanine World Hypothesis postulates that the evolution of the genetic code (the so-called GC phase) started with only four basic amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 a ...
s: alanine, glycine
Glycine (symbol Gly or G; ) is an amino acid that has a single hydrogen atom as its side chain. It is the simplest stable amino acid. Glycine is one of the proteinogenic amino acids. It is encoded by all the codons starting with GG (G ...
, proline and ornithine
Ornithine is a non-proteinogenic α-amino acid that plays a role in the urea cycle. It is not incorporated into proteins during translation. Ornithine is abnormally accumulated in the body in ornithine transcarbamylase deficiency, a disorder of th ...
(now arginine
Arginine is the amino acid with the formula (H2N)(HN)CN(H)(CH2)3CH(NH2)CO2H. The molecule features a guanidinium, guanidino group appended to a standard amino acid framework. At physiological pH, the carboxylic acid is deprotonated (−CO2−) a ...
). The evolution of the genetic code ended with 20 proteinogenic amino acids. From a chemical point of view, most of them are Alanine-derivatives particularly suitable for the construction of α-helices and β-sheets basic secondary structural elements of modern proteins. Direct evidence of this is an experimental procedure in molecular biology
Molecular biology is a branch of biology that seeks to understand the molecule, molecular basis of biological activity in and between Cell (biology), cells, including biomolecule, biomolecular synthesis, modification, mechanisms, and interactio ...
known as alanine scanning.
A hypothetical "Proline World" would create a possible alternative life with the genetic code based on the proline chemical scaffold as the protein backbone. Similarly, a "Glycine World" and "Ornithine World" are also conceivable, but nature has chosen none of them. Evolution of life
Life, also known as biota, refers to matter that has biological processes, such as Cell signaling, signaling and self-sustaining processes. It is defined descriptively by the capacity for homeostasis, Structure#Biological, organisation, met ...
with Proline, Glycine, or Ornithine as the basic structure for protein-like polymer
A polymer () is a chemical substance, substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeat unit, repeating subunits derived from one or more species of monomers. Due to their br ...
s ( foldamers) would lead to parallel biological worlds. They would have morphologically radically different body plan
A body plan, (), or ground plan is a set of morphology (biology), morphological phenotypic trait, features common to many members of a phylum of animals. The vertebrates share one body plan, while invertebrates have many.
This term, usually app ...
s and genetics
Genetics is the study of genes, genetic variation, and heredity in organisms.Hartl D, Jones E (2005) It is an important branch in biology because heredity is vital to organisms' evolution. Gregor Mendel, a Moravian Augustinians, Augustinian ...
from the living organisms of the known biosphere
The biosphere (), also called the ecosphere (), is the worldwide sum of all ecosystems. It can also be termed the zone of life on the Earth. The biosphere (which is technically a spherical shell) is virtually a closed system with regard to mat ...
.
Nonplanetary life
Dusty plasma-based
In 2007, Vadim N. Tsytovich and colleagues proposed that lifelike behaviors could be exhibited by dust particles suspended in a plasma, under conditions that might exist in space. Computer models showed that, when the dust became charged, the particles could self-organize into microscopic helical structures, and the authors offer "a rough sketch of a possible model of...helical grain structure reproduction".Cosmic necklace-based
In 2020, Luis A. Anchordoqu and Eugene M. Chudnovsky of theCity University of New York
The City University of New York (CUNY, pronounced , ) is the Public university, public university system of Education in New York City, New York City. It is the largest urban university system in the United States, comprising 25 campuses: eleven ...
hypothesized that cosmic necklace-based life composed of magnetic monopoles connected by cosmic strings could evolve inside stars. This would be achieved by a stretching of cosmic strings due to the star's intense gravity, thus allowing it to take on more complex forms and potentially form structures similar to the RNA and DNA structures found within carbon-based life. As such, it is theoretically possible that such beings could eventually become intelligent and construct a civilization using the power generated by the star's nuclear fusion. Because such use would use up part of the star's energy output, the luminosity would also fall. For this reason, it is thought that such life might exist inside stars observed to be cooling faster or dimmer than current cosmological models predict.
Life on a neutron star
Frank Drake suggested in 1973 that intelligent life could inhabitneutron star
A neutron star is the gravitationally collapsed Stellar core, core of a massive supergiant star. It results from the supernova explosion of a stellar evolution#Massive star, massive star—combined with gravitational collapse—that compresses ...
s. Physical models in 1973 implied that Drake's creatures would be microscopic.
Scientists who have published on this topic
Scientists who have considered possible alternatives to carbon-water biochemistry include: * J. B. S. Haldane (1892–1964), a geneticist noted for his work on abiogenesis. * V. Axel Firsoff (1910–1981), British astronomer. *Isaac Asimov
Isaac Asimov ( ; – April 6, 1992) was an Russian-born American writer and professor of biochemistry at Boston University. During his lifetime, Asimov was considered one of the "Big Three" science fiction writers, along with Robert A. H ...
(1920–1992), biochemist and science fiction writer.
*Fred Hoyle
Sir Fred Hoyle (24 June 1915 – 20 August 2001) was an English astronomer who formulated the theory of stellar nucleosynthesis and was one of the authors of the influential B2FH paper, B2FH paper. He also held controversial stances on oth ...
(1915–2001), astronomer and science fiction writer.
* Norman Horowitz (1915–2005), Caltech
The California Institute of Technology (branded as Caltech) is a private university, private research university in Pasadena, California, United States. The university is responsible for many modern scientific advancements and is among a small g ...
geneticist who devised the first experiments carried out to detect life on Mars.
* George C. Pimentel (1922–1989), American chemist, University of California, Berkeley
The University of California, Berkeley (UC Berkeley, Berkeley, Cal, or California), is a Public university, public Land-grant university, land-grant research university in Berkeley, California, United States. Founded in 1868 and named after t ...
.
* Peter Sneath (1923–2011), microbiologist, author of the book ''Planets and Life''.
* Gerald Feinberg (1933–1992), physicist and Robert Shapiro (1935–2011), chemist, co-authors of the book ''Life Beyond Earth''.
* Carl Sagan (1934–1996), astronomer, science popularizer, and SETI
Seti or SETI may refer to:
Astrobiology
* SETI, the search for extraterrestrial intelligence.
** SETI Institute, an astronomical research organization
*** SETIcon, a former convention organized by the SETI Institute
** Berkeley SETI Research Cent ...
proponent.
* Jonathan Lunine (born 1959), American planetary scientist and physicist.
* Robert Freitas (born 1952), specialist in nano-technology and nano-medicine.
* John Baross (born 1940), oceanographer and astrobiologist, who chaired a committee of scientists under the United States National Research Council that published a report on life's limiting conditions in 2007.Committee on the Limits of Organic Life in Planetary Systems, Committee on the Origins and Evolution of Life, National Research CouncilThe Limits of Organic Life in Planetary Systems
The National Academies Press, 2007.Committee on the Limits of Organic Life in Planetary Systems, Committee on the Origins and Evolution of Life, National Research Council
The Limits of Organic Life in Planetary Systems
The National Academies Press, 2007; page 5
See also
* Abiogenesis *Astrobiology
Astrobiology (also xenology or exobiology) is a scientific field within the List of life sciences, life and environmental sciences that studies the abiogenesis, origins, Protocell, early evolution, distribution, and future of life in the univ ...
* Carbon chauvinism
* Carbon-based life
Carbon is a primary component of all known life on Earth, and represents approximately 45–50% of all dry biomass. Carbon compounds occur naturally in great abundance on Earth. Complex biological molecules consist of carbon atoms bonded with ot ...
* Earliest known life forms
The earliest known life forms on Earth may be as old as 4.1 billion years (or Year#SI prefix multipliers, Ga) according to biologically fractionated graphite inside a single zircon grain in the Jack Hills range of Australia. The earliest evidenc ...
* Extraterrestrial life
Extraterrestrial life, or alien life (colloquially, aliens), is life that originates from another world rather than on Earth. No extraterrestrial life has yet been scientifically conclusively detected. Such life might range from simple forms ...
* Hachimoji DNA
* Iron–sulfur world hypothesis
* Life origination beyond planets
* Mirror life
* Nexus for Exoplanet System Science
* Non-cellular life
Non-cellular life, also known as acellular life, is life that exists without a cellular structure for at least part of its life cycle. Historically, most definitions of life postulated that an organism must be composed of one or more cells, ...
* Non-proteinogenic amino acids
* Nucleic acid analogues
* Planetary habitability
Planetary habitability is the measure of a planet's or a natural satellite's potential to Abiogenesis, develop and sustain an environment hospitable to life. Life may be abiogenesis, generated directly on a planet or satellite endogenously. Res ...
* Shadow biosphere
References
Further reading
*External links
Astronomy FAQ
{{Portal bar, Astronomy, Biology, Space Astrobiology Science fiction themes Biological hypotheses Scientific speculation