|
Silicon-based Life Form
Several forms of biochemistry are agreed to be scientifically viable but are not proven to exist at this time. The kinds of living organisms currently known on Earth all use carbon compounds for basic structural and metabolic functions, water as a solvent, and DNA or RNA to define and control their form. If life exists on other planets or moons it may be chemically similar, though it is also possible that there are organisms with quite different chemistries for instance, involving other classes of carbon compounds, compounds of another element, or another solvent in place of water. The possibility of life-forms being based on "alternative" biochemistries is the topic of an ongoing scientific discussion, informed by what is known about extraterrestrial environments and about the chemical behaviour of various elements and compounds. It is of interest in synthetic biology and is also a common subject in science fiction. The element silicon has been much discussed as a hypothetica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and Hydrophobe, hydrophobic; their odor is usually faint, and may be similar to that of gasoline or Naphtha, lighter fluid. They occur in a diverse range of molecular structures and phases: they can be gases (such as methane and propane), liquids (such as hexane and benzene), low melting solids (such as paraffin wax and naphthalene) or polymers (such as polyethylene and polystyrene). In the fossil fuel industries, ''hydrocarbon'' refers to naturally occurring petroleum, natural gas and coal, or their hydrocarbon derivatives and purified forms. Combustion of hydrocarbons is the main source of the world's energy. Petroleum is the dominant raw-material source for organic commodity chemicals such as solvents and polymers. Most anthropogenic (human-generated) emissions of greenhouse gases are eithe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Threose
Threose is a four-carbon monosaccharide with molecular formula C4H8O4. It has a terminal aldehyde group, rather than a ketone, in its linear chain and so is considered part of the aldose family of monosaccharides. The threose name can be used to refer to both the - and - stereoisomers and more generally to the racemic mixture (/L-, equal parts D- and L-) as well as to the more generic threose structure (absolute stereochemistry unspecified). The prefix "threo-" which derives from threose (and "erythro-" from a corresponding diastereomer erythrose) offer a useful way to describe general organic structures with adjacent chiral centers, where "the prefixes... designate the relative configuration of the centers".Formulas Using Other Configurational Notations W. Rausch, access ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
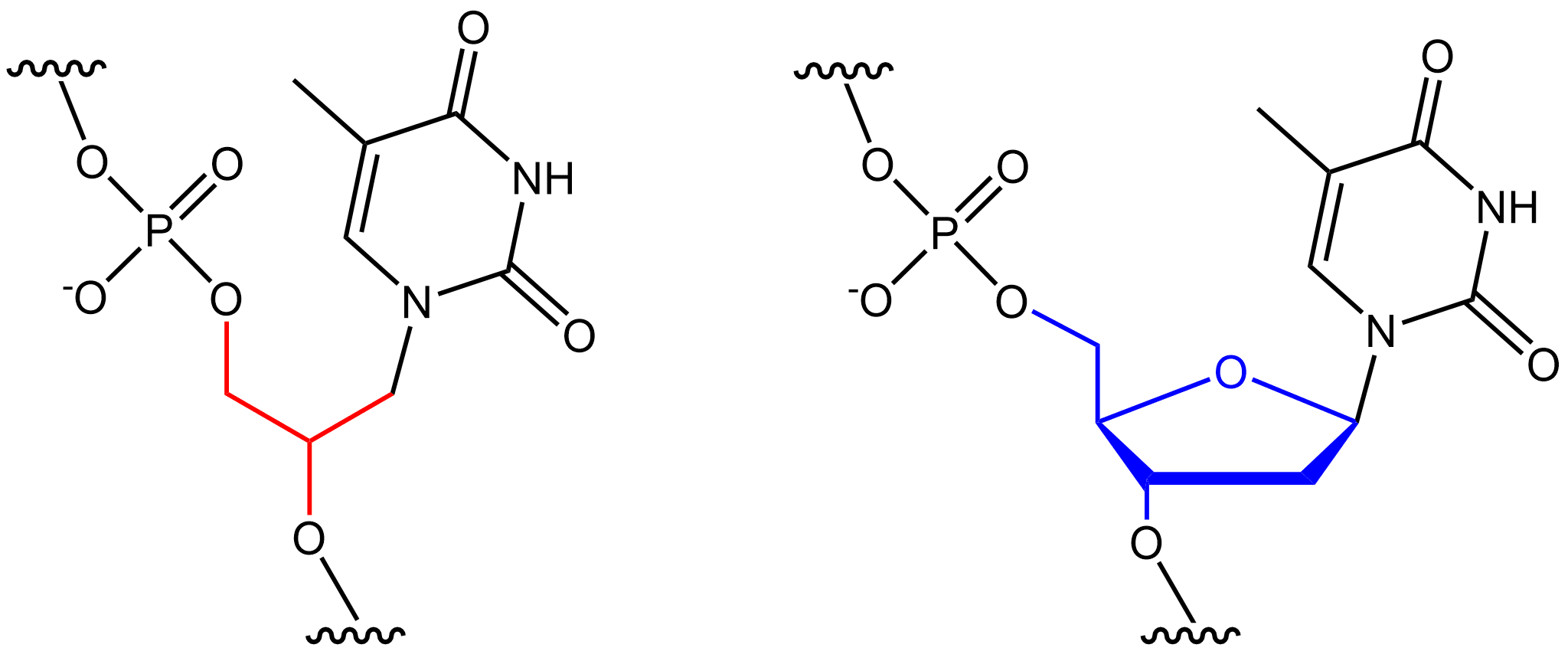
Threose Nucleic Acid
Threose nucleic acid (TNA) is an artificial genetic polymer in which the natural five-carbon ribose sugar found in RNA has been replaced by an unnatural four-carbon threose sugar.Schöning, K. U. ''et al.'' Chemical etiology of nucleic acid structure: the a-threofuranosyl-(3'-->2') oligonucleotide system. ''Science'' 290, 1347-1351, (2000) Invented by Albert Eschenmoser as part of his quest to explore the chemical etiology of RNA, TNA has become an important synthetic genetic polymer ( XNA) due to its ability to efficiently base pair with complementary sequences of DNA and RNA. The main difference between TNA and DNA/RNA is their backbones. DNA and RNA have their phosphate backbones attached to the 5' carbon of the deoxyribose or ribose sugar ring, respectively. TNA, on the other hand, has its phosphate backbone directly attached to the 3' carbon in the ring, since it does not have a 5' carbon. This modified backbone makes TNA, unlike DNA and RNA, completely refractory to nuclease d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Xeno Nucleic Acid
Xenonucleic acids (XNAs) are synthetic nucleic acid analogues that are engineered with structurally distinct components, such as alternative nucleosides, sugars, or backbones. XNAs have fundamentally different properties from endogenous nucleic acids, enabling different specialized applications, such as therapeutics, probes, or functional molecules. For instance, peptide nucleic acids, the backbones of which are made up of repeating aminoethylglycine units, are extremely stable and resistant to degradation by nucleases because they are not recognised. The same nucleobases can be used to store genetic information and interact with DNA, RNA, or other XNA bases, but the different backbone gives the compound different properties. Their altered chemical structure means they cannot be processed by naturally occurring cellular processes. For instance, natural DNA polymerases cannot read and duplicate the alien information, thus the genetic information stored in XNA is invisible to DNA- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Epimerase And Racemase
Epimerases and racemases are isomerase enzymes that catalyze the inversion of stereochemistry in biological molecules. Racemases catalyze the stereochemical inversion around the asymmetric carbon atom in a substrate having only one center of asymmetry. Epimerases catalyze the stereochemical inversion of the configuration about an asymmetric carbon atom in a substrate having more than one center of asymmetry, thus interconverting epimers. Human epimerases include methylmalonyl-CoA epimerase, involved in the metabolic breakdown of the amino acids alanine, isoleucine, methionine and valine Valine (symbol Val or V) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α- amino group (which is in the protonated −NH3+ form under biological conditions), an α- carboxylic acid group (which is in the deproton ..., and UDP-glucose 4-epimerase, which is used in the final step of galactose metabolism - catalyzing the reversible conversion of UDP-galactose to UD ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alanine
Alanine (symbol Ala or A), or α-alanine, is an α-amino acid that is used in the biosynthesis of proteins. It contains an amine group and a carboxylic acid group, both attached to the central carbon atom which also carries a methyl group side chain. Consequently it is classified as a non-polar, aliphatic α-amino acid. Under biological conditions, it exists in its zwitterionic form with its amine group protonated (as ) and its carboxyl group deprotonated (as ). It is non-essential to humans as it can be synthesized metabolically and does not need to be present in the diet. It is encoded by all codons starting with G C (GC U, GCC, GC A, and GCG). The L-isomer of alanine (left-handed) is the one that is incorporated into proteins. L-alanine is second only to L-leucine in rate of occurrence, accounting for 7.8% of the primary structure in a sample of 1,150 proteins. The right-handed form, D-alanine, occurs in peptides in some bacterial cell walls (in peptidoglycan) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
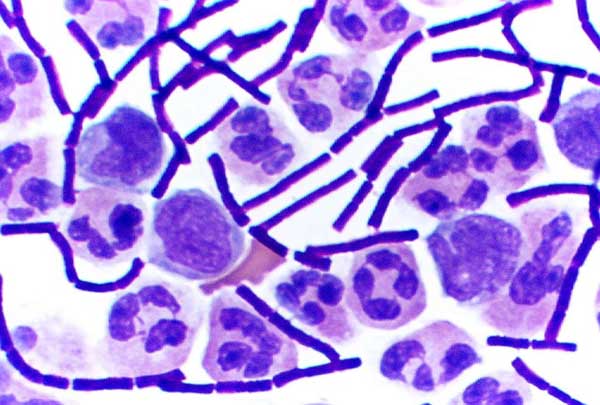
Gram-positive Bacteria
In bacteriology, gram-positive bacteria are bacteria that give a positive result in the Gram stain test, which is traditionally used to quickly classify bacteria into two broad categories according to their type of cell wall. The Gram stain is used by microbiologists to place bacteria into two main categories, gram-positive (+) and gram-negative (−). Gram-positive bacteria have a thick layer of peptidoglycan within the cell wall, and gram-negative bacteria have a thin layer of peptidoglycan. Gram-positive bacteria retain the crystal violet stain used in the test, resulting in a purple color when observed through an optical microscope. The thick layer of peptidoglycan in the bacterial cell wall retains the stain after it has been fixed in place by iodine. During the decolorization step, the decolorizer removes crystal violet from all other cells. Conversely, gram-negative bacteria cannot retain the violet stain after the decolorization step; alcohol used in this stage ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sugar
Sugar is the generic name for sweet-tasting, soluble carbohydrates, many of which are used in food. Simple sugars, also called monosaccharides, include glucose Glucose is a sugar with the Chemical formula#Molecular formula, molecular formula , which is often abbreviated as Glc. It is overall the most abundant monosaccharide, a subcategory of carbohydrates. It is mainly made by plants and most algae d ..., fructose, and galactose. Compound sugars, also called disaccharides or double sugars, are molecules made of two bonded monosaccharides; common examples are sucrose (glucose + fructose), lactose (glucose + galactose), and maltose (two molecules of glucose). White sugar is almost pure sucrose. In the body, compound sugars are hydrolysed into simple sugars. Longer chains of monosaccharides (>2) are not regarded as sugars and are called oligosaccharides or polysaccharides. Starch is a glucose polymer found in plants, the most abundant source of energy in human foo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 appear in the genetic code of life. Amino acids can be classified according to the locations of the core structural functional groups ( alpha- , beta- , gamma- amino acids, etc.); other categories relate to polarity, ionization, and side-chain group type ( aliphatic, acyclic, aromatic, polar, etc.). In the form of proteins, amino-acid '' residues'' form the second-largest component (water being the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis. It is thought that they played a key role in enabling life on Earth and its emergence. Amino acids are formally named by the IUPAC- IUBMB Joint Commi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Left-handed Protein
In chemistry, a molecule or ion is called chiral () if it cannot be superposed on its mirror image by any combination of rotations, translations, and some conformational changes. This geometric property is called chirality (). The terms are derived from Ancient Greek (''cheir'') 'hand'; which is the canonical example of an object with this property. A chiral molecule or ion exists in two stereoisomers that are mirror images of each other, called enantiomers; they are often distinguished as either "right-handed" or "left-handed" by their absolute configuration or some other criterion. The two enantiomers have the same chemical properties, except when reacting with other chiral compounds. They also have the same physical properties, except that they often have opposite optical activities. A homogeneous mixture of the two enantiomers in equal parts is said to be racemic, and it usually differs chemically and physically from the pure enantiomers. Chiral molecules will usually h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chirality
Chirality () is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object. An object or a system is ''chiral'' if it is distinguishable from its mirror image; that is, it cannot be superposed (not to be confused with superimposed) onto it. Conversely, a mirror image of an ''achiral'' object, such as a sphere, cannot be distinguished from the object. A chiral object and its mirror image are called '' enantiomorphs'' (Greek, "opposite forms") or, when referring to molecules, ''enantiomers''. A non-chiral object is called ''achiral'' (sometimes also ''amphichiral'') and can be superposed on its mirror image. The term was first used by Lord Kelvin in 1893 in the second Robert Boyle Lecture at the Oxford University Junior Scientific Club which was published in 1894: Human hands are perhaps the most recognized example of chirality. The left hand is a non-superposable mirror ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |