|
Ornithine
Ornithine is a non-proteinogenic α-amino acid that plays a role in the urea cycle. It is not incorporated into proteins during translation. Ornithine is abnormally accumulated in the body in ornithine transcarbamylase deficiency, a disorder of the urea cycle. The Moiety (chemistry), moiety derived from ornithine is called ornithyl. Role in urea cycle L-Ornithine is one of the products of the action of the enzyme arginase on L-arginine, creating urea. Therefore, ornithine is a central component of the urea cycle, which enables the disposal of excess nitrogen. Ornithine itself is recycled and, in a sense, acts as a catalyst. First, ammonia is converted into carbamoyl phosphate () by carbamoyl phosphate synthetase. Ornithine transcarbamylase then catalyzes the reaction between carbamoyl phosphate and ornithine to form citrulline and phosphate (Pi). Another amino group is contributed by aspartate, leading to the formation of arginine and the byproduct fumarate. The resulting arginine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ornithine Transcarbamylase Deficiency
Ornithine transcarbamylase deficiency also known as OTC deficiency is the most common urea cycle disorder in humans. Ornithine transcarbamylase, the defective enzyme in this disorder, is the final enzyme in the proximal portion of the urea cycle, responsible for converting carbamoyl phosphate and ornithine into citrulline. OTC deficiency is inherited in an X-linked recessive manner, meaning males are more commonly affected than females. In severely affected individuals, hyperammonemia, ammonia concentrations increase rapidly causing ataxia, lethargy and death without rapid intervention. OTC deficiency is diagnosed using a combination of clinical findings and biochemical testing, while confirmation is often done using molecular genetics techniques. Once an individual has been diagnosed, the treatment goal is to avoid precipitating episodes that can cause an increased ammonia concentration. The most common treatment combines a low protein diet with nitrogen scavenging agents. Liver t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ornithine Lactamization
Ornithine is a non-proteinogenic α-amino acid that plays a role in the urea cycle. It is not incorporated into proteins during translation. Ornithine is abnormally accumulated in the body in ornithine transcarbamylase deficiency, a disorder of the urea cycle. The moiety derived from ornithine is called ornithyl. Role in urea cycle L-Ornithine is one of the products of the action of the enzyme arginase on L-arginine, creating urea. Therefore, ornithine is a central component of the urea cycle, which enables the disposal of excess nitrogen. Ornithine itself is recycled and, in a sense, acts as a catalyst. First, ammonia is converted into carbamoyl phosphate () by carbamoyl phosphate synthetase. Ornithine transcarbamylase then catalyzes the reaction between carbamoyl phosphate and ornithine to form citrulline and phosphate (Pi). Another amino group is contributed by aspartate, leading to the formation of arginine and the byproduct fumarate. The resulting arginine, a guanidinium com ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ornithine Transcarbamylase
Ornithine transcarbamylase (OTC) (also called ornithine carbamoyltransferase) is an enzyme () that catalyzes the reaction between carbamoyl phosphate (CP) and ornithine (Orn) to form citrulline (Cit) and phosphate (Pi). There are two classes of OTC: anabolic and catabolic. This article focuses on anabolic OTC. Anabolic OTC facilitates the sixth step in the biosynthesis of the amino acid arginine in prokaryotes. In contrast, mammalian OTC plays an essential role in the urea cycle, the purpose of which is to capture toxic ammonia and transform it into urea, a less toxic nitrogen source, for excretion. Reaction mechanism Structure OTC is a trimeric protein. There are three active sites of the protein which are located at the cleft between the monomers. The carbamoyl phosphate binding domain resides on the N-terminal end of each monomer, while the C-terminal end contains the binding domain for ornithine. Both binding domains have a similar structural pattern with a central para ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ornithine Decarboxylase
The enzyme ornithine decarboxylase (, ODC) catalyzes the decarboxylation of ornithine (a product of the urea cycle) to form putrescine. This reaction is the committed step in polyamine synthesis. In humans, this protein has 461 amino acids and forms a homodimer. In humans, ornithine decarboxylase (ODC) is expressed by the gene ''ODC1''. The protein ODC is sometimes referred to as "ODC1" in research pertaining to humans and mice, but certain species such as ''Drosophila'' (''dODC2''), species of Solanaceae plant family (''ODC2''), and the lactic acid bacteria '' Paucilactobacillus wasatchensis'' (''odc2'') have been shown to have a second ODC gene. Reaction mechanism Lysine 69 on ornithine decarboxylase (ODC) binds the cofactor pyridoxal phosphate to form a Schiff base. Ornithine displaces the lysine to form a Schiff base attached to orthonine, which decarboxylates to form a quinoid intermediate. This intermediate rearranges to form a Schiff base attached to putrescine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Urea Cycle
The urea cycle (also known as the ornithine cycle) is a cycle of biochemical reactions that produces urea (NH2)2CO from ammonia (NH3). Animals that use this cycle, mainly amphibians and mammals, are called ureotelic. The urea cycle converts highly toxic ammonia to urea for excretion. This cycle was the first metabolic cycle to be discovered by Hans Krebs and Kurt Henseleit in 1932, five years before the discovery of the TCA cycle. The urea cycle was described in more detail later on by Ratner and Cohen. The urea cycle takes place primarily in the liver and, to a lesser extent, in the kidneys. Function Amino acid catabolism results in waste ammonia. All animals need a way to excrete this product. Most aquatic organisms, or ammonotelic organisms, excrete ammonia without converting it. Organisms that cannot easily and safely remove nitrogen as ammonia convert it to a less toxic substance, such as urea, via the urea cycle, which occurs mainly in the liver. Urea produced by the li ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bisorcic
Bisorcic (), also known as ''N''2,''N''5-diacetyl-L-ornithine, is a drug described as a hepatoprotective agent and "psychostimulant" which has been used in France in the treatment of asthenia. It is the ''N''2,''N''5- diacetylated derivative of the amino acid L-ornithine. Bisorcic was first described in the literature in 1973 in a German patent. The was designated around 1975. The drug was marketed in France by Astyl-Gallier in 1987. It was provided in the form of 200mg oral capsules and four capsules were taken daily. L-Ornithine, as the combination drug L-ornithine L-aspartate (LOLA), has been used in the treatment of hepatic encephalopathy and cirrhosis and is likewise described as hepatoprotective. It is thought to work by participating in the urea cycle and lowering ammonia Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hepatic Encephalopathy
Hepatic encephalopathy (HE) is an altered level of consciousness as a result of liver failure. Its onset may be gradual or sudden. Other symptoms may include movement problems, changes in mood, or changes in personality. In the advanced stages, it can result in a coma. Hepatic encephalopathy can occur in those with acute or chronic liver disease. Episodes can be triggered by infections, gastrointestinal bleeding, constipation, electrolyte problems, or certain medications. The underlying mechanism is believed to involve the buildup of ammonia in the blood, a substance that is normally removed by the liver. The diagnosis is typically based on symptoms after ruling out other potential causes. It may be supported by blood ammonia levels, an electroencephalogram, or computer tomography (CT scan) of the brain. Hepatic encephalopathy is possibly reversible with treatment. This typically involves supportive care and addressing the triggers of the event. Lactulose is fre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha-Amino Acids
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 appear in the genetic code of life. Amino acids can be classified according to the locations of the core structural functional groups ( alpha- , beta- , gamma- amino acids, etc.); other categories relate to polarity, ionization, and side-chain group type ( aliphatic, acyclic, aromatic, polar, etc.). In the form of proteins, amino-acid '' residues'' form the second-largest component (water being the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis. It is thought that they played a key role in enabling life on Earth and its emergence. Amino acids are formally named by the IUPAC- IUBMB Joint Commiss ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbamoyl Phosphate
Carbamoyl phosphate is an anion of biochemical significance. In land-dwelling animals, it is an intermediary metabolite in nitrogen disposal through the urea cycle and the synthesis of pyrimidines. Its enzymatic counterpart, carbamoyl phosphate synthetase I (CPS I), interacts with a class of molecules called sirtuins, NAD dependent protein deacetylases, and ATP to form carbamoyl phosphate. CP then enters the urea cycle in which it reacts with ornithine (a process catalyzed by the enzyme ornithine transcarbamylase) to form citrulline. Classification Carbamoyl phosphate is a metabolic intermediate in a pathway that involves nitrogen disposal through the urea cycle and the biosynthesis of pyrimidines. Production It is produced from bicarbonate, ammonia (derived from amino acids), and phosphate (from ATP). The synthesis is catalyzed by the enzyme carbamoyl phosphate synthetase. This uses three reactions as follows: * + ATP → ADP + (carboxyl phosphate) * + NH3 + OH− ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
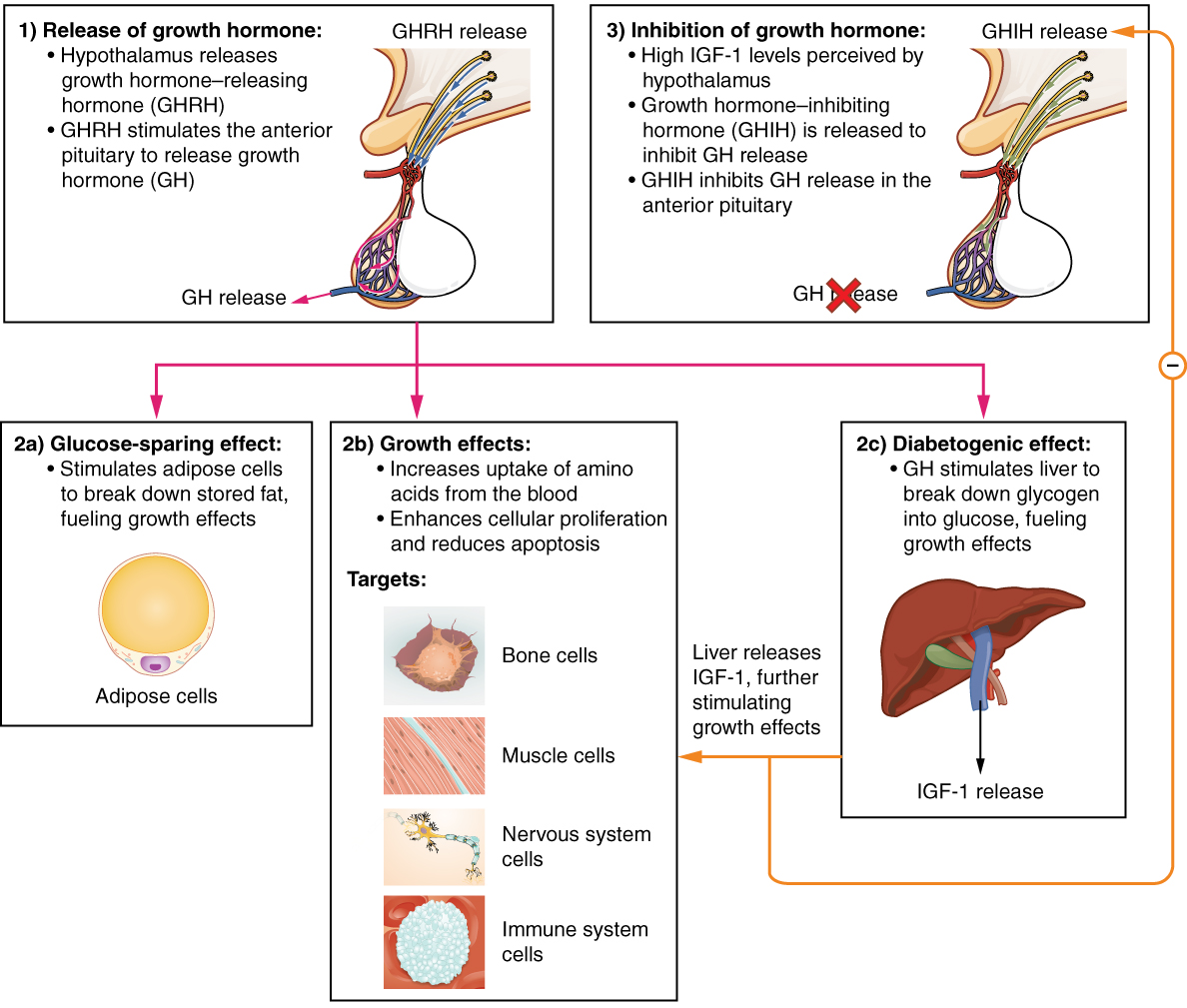
Human Growth Hormone
Growth hormone (GH) or somatotropin, also known as human growth hormone (hGH or HGH) in its human form, is a peptide hormone that stimulates growth, cell reproduction, and cell regeneration in humans and other animals. It is thus important in human development. GH also stimulates production of insulin-like growth factor 1 (IGF-1) and increases the concentration of glucose and free fatty acids. It is a type of mitogen which is specific only to the receptors on certain types of cells. GH is a 191-amino acid, single-chain polypeptide that is synthesized, stored and secreted by somatotropic cells within the lateral wings of the anterior pituitary gland. A recombinant form of HGH called somatropin ( INN) is used as a prescription drug to treat children's growth disorders and adult growth hormone deficiency. In the United States, it is only available legally from pharmacies by prescription from a licensed health care provider. In recent years in the United States, some health ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3-Methylornithine
3-Methylornithine is an amino acid with the formula H2N(CH2)2CH(CH3)CH(NH2)CO2H. This amino acid contains two stereogenic centers, but only one stereoisomer (namely (3''R'')-3-methyl-D-ornithine) occurs in nature. It is produced from lysine by the action of the enzyme methylornithine synthase. The combination of lysine and 3-methylornithine, also mediated enzymatically, produces pyrrolysine, which, for some organisms, is a 22nd genetically coded amino acid.Quitterer, F.; Beck, P.; Bacher, A.; Groll, M., "Structure and Reaction Mechanism of Pyrrolysine Synthase (PylD)", Angew. Chem. Int. Ed. 2013, volume 52, pp. 7033-7037. See also * Ornithine Ornithine is a non-proteinogenic α-amino acid that plays a role in the urea cycle. It is not incorporated into proteins during translation. Ornithine is abnormally accumulated in the body in ornithine transcarbamylase deficiency, a disorder of th ... References {{DEFAULTSORT:Methylornithine, 3- Alpha-Amino acids Basic amino aci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |