reversible inhibition on:
[Wikipedia]
[Google]
[Amazon]
 An enzyme inhibitor is a
An enzyme inhibitor is a
 An enzyme inhibitor is characterised by its
An enzyme inhibitor is characterised by its
 Not all irreversible inhibitors form covalent adducts with their enzyme targets. Some reversible inhibitors bind so tightly to their target enzyme that they are essentially irreversible. These tight-binding inhibitors may show kinetics similar to covalent irreversible inhibitors. In these cases some of these inhibitors rapidly bind to the enzyme in a low-affinity EI complex and this then undergoes a slower rearrangement to a very tightly bound EI* complex (see the "irreversible inhibition mechanism" diagram). This kinetic behaviour is called slow-binding. This slow rearrangement after binding often involves a
Not all irreversible inhibitors form covalent adducts with their enzyme targets. Some reversible inhibitors bind so tightly to their target enzyme that they are essentially irreversible. These tight-binding inhibitors may show kinetics similar to covalent irreversible inhibitors. In these cases some of these inhibitors rapidly bind to the enzyme in a low-affinity EI complex and this then undergoes a slower rearrangement to a very tightly bound EI* complex (see the "irreversible inhibition mechanism" diagram). This kinetic behaviour is called slow-binding. This slow rearrangement after binding often involves a
 Diisopropylfluorophosphate (DFP) is an example of an irreversible protease inhibitor (see the "DFP reaction" diagram). The enzyme hydrolyses the phosphorus–fluorine bond, but the phosphate residue remains bound to the serine in the
Diisopropylfluorophosphate (DFP) is an example of an irreversible protease inhibitor (see the "DFP reaction" diagram). The enzyme hydrolyses the phosphorus–fluorine bond, but the phosphate residue remains bound to the serine in the
 Animals and plants have evolved to synthesise a vast array of poisonous products including
Animals and plants have evolved to synthesise a vast array of poisonous products including

 The most common uses for enzyme inhibitors are as drugs to treat disease. Many of these inhibitors target a human enzyme and aim to correct a pathological condition. For instance,
The most common uses for enzyme inhibitors are as drugs to treat disease. Many of these inhibitors target a human enzyme and aim to correct a pathological condition. For instance,
 Drugs are also used to inhibit enzymes needed for the survival of pathogens. For example, bacteria are surrounded by a thick
Drugs are also used to inhibit enzymes needed for the survival of pathogens. For example, bacteria are surrounded by a thick
 New drugs are the products of a long
New drugs are the products of a long
molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
that binds to an enzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different mol ...
and blocks its activity. Enzymes are protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
s that speed up chemical reaction
A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, ...
s necessary for life
Life, also known as biota, refers to matter that has biological processes, such as Cell signaling, signaling and self-sustaining processes. It is defined descriptively by the capacity for homeostasis, Structure#Biological, organisation, met ...
, in which substrate molecules are converted into products. An enzyme facilitates a specific chemical reaction by binding the substrate to its active site
In biology and biochemistry, the active site is the region of an enzyme where substrate molecules bind and undergo a chemical reaction. The active site consists of amino acid residues that form temporary bonds with the substrate, the ''binding s ...
, a specialized area on the enzyme that accelerates the most difficult step of the reaction.
An enzyme inhibitor stops ("inhibits") this process, either by binding to the enzyme's active site (thus preventing the substrate itself from binding) or by binding to another site on the enzyme such that the enzyme's catalysis
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
of the reaction is blocked. Enzyme inhibitors may bind reversibly or irreversibly. Irreversible inhibitors form a chemical bond
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds or through the sharing of electrons a ...
with the enzyme such that the enzyme is inhibited until the chemical bond is broken. By contrast, reversible inhibitors bind non-covalently and may spontaneously leave the enzyme, allowing the enzyme to resume its function. Reversible inhibitors produce different types of inhibition depending on whether they bind to the enzyme, the enzyme-substrate complex, or both.
Enzyme inhibitors play an important role in all cells, since they are generally specific to one enzyme each and serve to control that enzyme's activity. For example, enzymes in a metabolic pathway
In biochemistry, a metabolic pathway is a linked series of chemical reactions occurring within a cell (biology), cell. The reactants, products, and Metabolic intermediate, intermediates of an enzymatic reaction are known as metabolites, which are ...
may be inhibited by molecules produced later in the pathway, thus curtailing the production of molecules that are no longer needed. This type of negative feedback
Negative feedback (or balancing feedback) occurs when some function (Mathematics), function of the output of a system, process, or mechanism is feedback, fed back in a manner that tends to reduce the fluctuations in the output, whether caused ...
is an important way to maintain balance
Balance may refer to:
Common meanings
* Balance (ability) in biomechanics
* Balance (accounting)
* Balance or weighing scale
* Balance, as in equality (mathematics) or equilibrium
Arts and entertainment Film
* Balance (1983 film), ''Balance'' ( ...
in a cell. Enzyme inhibitors also control essential enzymes such as protease
A protease (also called a peptidase, proteinase, or proteolytic enzyme) is an enzyme that catalysis, catalyzes proteolysis, breaking down proteins into smaller polypeptides or single amino acids, and spurring the formation of new protein products ...
s or nuclease
In biochemistry, a nuclease (also archaically known as nucleodepolymerase or polynucleotidase) is an enzyme capable of cleaving the phosphodiester bonds that link nucleotides together to form nucleic acids. Nucleases variously affect single and ...
s that, if left unchecked, may damage a cell. Many poison
A poison is any chemical substance that is harmful or lethal to living organisms. The term is used in a wide range of scientific fields and industries, where it is often specifically defined. It may also be applied colloquially or figurati ...
s produced by animals or plants are enzyme inhibitors that block the activity of crucial enzymes in prey or predator
Predation is a biological interaction in which one organism, the predator, kills and eats another organism, its prey. It is one of a family of common List of feeding behaviours, feeding behaviours that includes parasitism and micropredation ...
s.
Many drug molecules are enzyme inhibitors that inhibit an aberrant human enzyme or an enzyme critical for the survival of a pathogen
In biology, a pathogen (, "suffering", "passion" and , "producer of"), in the oldest and broadest sense, is any organism or agent that can produce disease. A pathogen may also be referred to as an infectious agent, or simply a Germ theory of d ...
such as a virus
A virus is a submicroscopic infectious agent that replicates only inside the living Cell (biology), cells of an organism. Viruses infect all life forms, from animals and plants to microorganisms, including bacteria and archaea. Viruses are ...
, bacterium
Bacteria (; : bacterium) are ubiquitous, mostly free-living organisms often consisting of one biological cell. They constitute a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria were among the ...
or parasite
Parasitism is a Symbiosis, close relationship between species, where one organism, the parasite, lives (at least some of the time) on or inside another organism, the Host (biology), host, causing it some harm, and is Adaptation, adapted str ...
. Examples include methotrexate
Methotrexate, formerly known as amethopterin, is a chemotherapy agent and immunosuppressive drug, immune-system suppressant. It is used to treat cancer, autoimmune diseases, and ectopic pregnancy, ectopic pregnancies. Types of cancers it is u ...
(used in chemotherapy
Chemotherapy (often abbreviated chemo, sometimes CTX and CTx) is the type of cancer treatment that uses one or more anti-cancer drugs (list of chemotherapeutic agents, chemotherapeutic agents or alkylating agents) in a standard chemotherapy re ...
and in treating rheumatic arthritis) and the protease inhibitors
Protease inhibitors (PIs) are medications that act by interfering with protease, enzymes that cleave proteins. Some of the most well known are antiviral drugs widely used to treat HIV/AIDS, hepatitis C and COVID-19. These protease inhibitors pre ...
used to treat HIV/AIDS
The HIV, human immunodeficiency virus (HIV) is a retrovirus that attacks the immune system. Without treatment, it can lead to a spectrum of conditions including acquired immunodeficiency syndrome (AIDS). It is a Preventive healthcare, pr ...
. Since anti-pathogen inhibitors generally target only one enzyme, such drugs are highly specific
Specific may refer to:
* Specificity (disambiguation)
* Specific, a cure or therapy for a specific illness
Law
* Specific deterrence, focussed on an individual
* Specific finding, intermediate verdict used by a jury in determining the final ...
and generally produce few side effects in humans, provided that no analogous
Analogy is a comparison or correspondence between two things (or two groups of things) because of a third element that they are considered to share.
In logic, it is an inference or an argument from one particular to another particular, as oppose ...
enzyme is found in humans. (This is often the case, since such pathogen
In biology, a pathogen (, "suffering", "passion" and , "producer of"), in the oldest and broadest sense, is any organism or agent that can produce disease. A pathogen may also be referred to as an infectious agent, or simply a Germ theory of d ...
s and human
Humans (''Homo sapiens'') or modern humans are the most common and widespread species of primate, and the last surviving species of the genus ''Homo''. They are Hominidae, great apes characterized by their Prehistory of nakedness and clothing ...
s are genetically distant.) Medicinal enzyme inhibitors often have low dissociation constant
In chemistry, biochemistry, and pharmacology, a dissociation constant (''K''D) is a specific type of equilibrium constant that measures the propensity of a larger object to separate (dissociate) reversibly into smaller components, as when a complex ...
s, meaning that only a minute amount of the inhibitor is required to inhibit the enzyme. A low concentration of the enzyme inhibitor reduces the risk for liver
The liver is a major metabolic organ (anatomy), organ exclusively found in vertebrates, which performs many essential biological Function (biology), functions such as detoxification of the organism, and the Protein biosynthesis, synthesis of var ...
and kidney damage and other adverse drug reaction
An adverse drug reaction (ADR) is a harmful, unintended result caused by taking medication. ADRs may occur following a single dose or prolonged administration of a drug or may result from the combination of two or more drugs. The meaning of this ...
s in humans. Hence the discovery and refinement of enzyme inhibitors is an active area of research in biochemistry
Biochemistry, or biological chemistry, is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology, a ...
and pharmacology
Pharmacology is the science of drugs and medications, including a substance's origin, composition, pharmacokinetics, pharmacodynamics, therapeutic use, and toxicology. More specifically, it is the study of the interactions that occur betwee ...
.
Structural classes
Enzyme inhibitors are a chemically diverse set of substances that range in size from organicsmall molecule
In molecular biology and pharmacology, a small molecule or micromolecule is a low molecular weight (≤ 1000 daltons) organic compound that may regulate a biological process, with a size on the order of 1 nm. Many drugs are small molecules; ...
s to macromolecular protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
s.
Small molecule inhibitors include essential primary metabolite
A primary metabolite is a kind of metabolite that is directly involved in normal growth, development, and reproduction. It usually performs a physiological function in the organism (i.e. an intrinsic function). A primary metabolite is typically pre ...
s that inhibit upstream enzymes that produce those metabolites. This provides a negative feedback loop that prevents over production of metabolites and thus maintains cellular homeostasis
In biology, homeostasis (British English, British also homoeostasis; ) is the state of steady internal physics, physical and chemistry, chemical conditions maintained by organism, living systems. This is the condition of optimal functioning fo ...
(steady internal conditions). Small molecule enzyme inhibitors also include secondary metabolite
Secondary metabolites, also called ''specialised metabolites'', ''secondary products'', or ''natural products'', are organic compounds produced by any lifeform, e.g. bacteria, archaea, fungi, animals, or plants, which are not directly involved ...
s, which are not essential to the organism that produces them, but provide the organism with an evolutionary advantage, in that they can be used to repel predators or competing organisms or immobilize prey. In addition, many drugs are small molecule enzyme inhibitors that target either disease-modifying enzymes in the patient or enzymes in pathogens which are required for the growth and reproduction of the pathogen.
In addition to small molecules, some proteins act as enzyme inhibitors. The most prominent example are serpin
Serpins are a superfamily of proteins with similar structures that were first identified for their protease inhibition activity and are found in all kingdoms of life. The acronym serpin was originally coined because the first serpins to be ...
s (serine protease inhibitors) which are produced by animals to protect against inappropriate enzyme activation and by plants to prevent predation. Another class of inhibitor proteins is the ribonuclease inhibitor
Ribonuclease inhibitor (RI) is a large (~450 residues, ~49 kDa), acidic (pI ~4.7), leucine-rich repeat protein that forms extremely tight complexes with certain ribonucleases. It is a major cellular protein, comprising ~0.1% of all cellular prote ...
s, which bind to ribonuclease
Ribonuclease (commonly abbreviated RNase) is a type of nuclease that catalyzes the degradation of RNA into smaller components. Ribonucleases can be divided into endoribonucleases and exoribonucleases, and comprise several sub-classes within th ...
s in one of the tightest known protein–protein interaction
Protein–protein interactions (PPIs) are physical contacts of high specificity established between two or more protein molecules as a result of biochemical events steered by interactions that include electrostatic forces, hydrogen bonding and t ...
s. A special case of protein enzyme inhibitors are zymogen
In biochemistry, a zymogen (), also called a proenzyme (), is an inactive precursor of an enzyme. A zymogen requires a biochemical change (such as a hydrolysis reaction revealing the active site, or changing the configuration to reveal the activ ...
s that contain an autoinhibitory N-terminal
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the amin ...
peptide that binds to the active site of enzyme that intramolecularly blocks its activity as a protective mechanism against uncontrolled catalysis. The Nterminal peptide is cleaved (split) from the zymogen enzyme precursor by another enzyme to release an active enzyme.
The binding site
In biochemistry and molecular biology, a binding site is a region on a macromolecule such as a protein that binds to another molecule with specificity. The binding partner of the macromolecule is often referred to as a ligand. Ligands may includ ...
of inhibitors on enzymes is most commonly the same site that binds the substrate
Substrate may refer to:
Physical layers
*Substrate (biology), the natural environment in which an organism lives, or the surface or medium on which an organism grows or is attached
** Substrate (aquatic environment), the earthy material that exi ...
of the enzyme. These active site
In biology and biochemistry, the active site is the region of an enzyme where substrate molecules bind and undergo a chemical reaction. The active site consists of amino acid residues that form temporary bonds with the substrate, the ''binding s ...
inhibitors are known as orthosteric ("regular" orientation) inhibitors. The mechanism of orthosteric inhibition is simply to prevent substrate binding to the enzyme through direct competition which in turn prevents the enzyme from catalysing the conversion of substrates into products. Alternatively, the inhibitor can bind to a site remote from the enzyme active site. These are known as allosteric
In the fields of biochemistry and pharmacology an allosteric regulator (or allosteric modulator) is a substance that binds to a site on an enzyme or receptor distinct from the active site, resulting in a conformational change that alters the p ...
("alternative" orientation) inhibitors. The mechanisms of allosteric inhibition are varied and include changing the conformation (shape) of the enzyme such that it can no longer bind substrate ( kinetically indistinguishable from competitive orthosteric inhibition) or alternatively stabilise binding of substrate to the enzyme but lock the enzyme in a conformation which is no longer catalytically active.
Reversible inhibitors
Reversible inhibitors attach to enzymes with non-covalent interactions such ashydrogen bond
In chemistry, a hydrogen bond (H-bond) is a specific type of molecular interaction that exhibits partial covalent character and cannot be described as a purely electrostatic force. It occurs when a hydrogen (H) atom, Covalent bond, covalently b ...
s, hydrophobic interaction
In chemistry, hydrophobicity is the chemical property of a molecule (called a hydrophobe) that is seemingly repelled from a mass of water. In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to be nonpolar and, thus, ...
s and ionic bond
Ionic bonding is a type of chemical bond
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic ...
s. Multiple weak bonds between the inhibitor and the enzyme active site
In biology and biochemistry, the active site is the region of an enzyme where substrate molecules bind and undergo a chemical reaction. The active site consists of amino acid residues that form temporary bonds with the substrate, the ''binding s ...
combine to produce strong and specific binding.
In contrast to irreversible inhibitors, reversible inhibitors generally do not undergo chemical reactions when bound to the enzyme and can be easily removed by dilution or dialysis. A special case is covalent reversible inhibitors that form a chemical bond with the enzyme, but the bond can be cleaved so the inhibition is fully reversible.
Reversible inhibitors are generally categorized into four types, as introduced by Cleland in 1963. They are classified according to the effect of the inhibitor on the ''Vmax'' (maximum reaction rate catalysed by the enzyme) and ''Km'' (the concentration of substrate resulting in half maximal enzyme activity) as the concentration of the enzyme's substrate is varied.
Competitive
Incompetitive inhibition
Competitive inhibition is interruption of a chemistry, chemical pathway owing to one chemical substance inhibiting the effect of another by competing with it for molecular binding, binding or chemical bond, bonding. Any metabolism, metabolic or c ...
the substrate and inhibitor cannot bind to the enzyme at the same time. This usually results from the inhibitor having an affinity for the active site of an enzyme where the substrate also binds; the substrate and inhibitor ''compete'' for access to the enzyme's active site. This type of inhibition can be overcome by sufficiently high concentrations of substrate (''Vmax'' remains constant), i.e., by out-competing the inhibitor. However, the apparent ''Km'' will increase as it takes a higher concentration of the substrate to reach the ''Km'' point, or half the ''Vmax''. Competitive inhibitors are often similar in structure to the real substrate (see for example the "methotrexate versus folate" figure in the "Drugs" section).
Uncompetitive
In uncompetitive inhibition the inhibitor binds only to the enzyme-substrate complex. This type of inhibition causes ''Vmax'' to decrease (maximum velocity decreases as a result of removing activated complex) and ''Km'' to decrease (due to better binding efficiency as a result ofLe Chatelier's principle
In chemistry, Le Chatelier's principle (pronounced or ) is a principle used to predict the effect of a change in conditions on chemical equilibrium. Other names include Chatelier's principle, Braun–Le Chatelier principle, Le Chatelier–Braun p ...
and the effective elimination of the ES complex thus decreasing the ''Km'' which indicates a higher binding affinity). Uncompetitive inhibition is rare.
Non-competitive
Innon-competitive inhibition
Non-competitive inhibition is a type of enzyme inhibition where the inhibitor reduces the activity of the enzyme and binds equally well to the enzyme regardless of whether it has already bound the substrate. This is unlike competitive inhibition ...
the binding of the inhibitor to the enzyme reduces its activity but does not affect the binding of substrate. This type of inhibitor binds with equal affinity to the free enzyme as to the enzyme-substrate complex. It can be thought of as having the ability of competitive and uncompetitive inhibitors, but with no preference to either type. As a result, the extent of inhibition depends only on the concentration of the inhibitor. ''Vmax'' will decrease due to the inability for the reaction to proceed as efficiently, but ''Km'' will remain the same as the actual binding of the substrate, by definition, will still function properly.
Mixed
In mixed inhibition the inhibitor may bind to the enzyme whether or not the substrate has already bound. Hence mixed inhibition is a combination of competitive and noncompetitive inhibition. Furthermore, the affinity of the inhibitor for the free enzyme and the enzyme-substrate complex may differ. By increasing concentrations of substrate this type of inhibition can be reduced (due to the competitive contribution), but not entirely overcome (due to the noncompetitive component). Although it is possible for mixed-type inhibitors to bind in the active site, this type of inhibition generally results from anallosteric
In the fields of biochemistry and pharmacology an allosteric regulator (or allosteric modulator) is a substance that binds to a site on an enzyme or receptor distinct from the active site, resulting in a conformational change that alters the p ...
effect where the inhibitor binds to a different site on an enzyme. Inhibitor binding to this allosteric site changes the conformation (that is, the tertiary structure
Protein tertiary structure is the three-dimensional shape of a protein. The tertiary structure will have a single polypeptide chain "backbone" with one or more protein secondary structures, the protein domains. Amino acid side chains and the ...
or three-dimensional shape) of the enzyme so that the affinity of the substrate for the active site is reduced.
These four types of inhibition can also be distinguished by the effect of increasing the substrate concentration on the degree of inhibition caused by a given amount of inhibitor. For competitive inhibition the degree of inhibition is reduced by increasing for noncompetitive inhibition the degree of inhibition is unchanged, and for uncompetitive (also called anticompetitive) inhibition the degree of inhibition increases with
Quantitative description
Reversible inhibition can be described quantitatively in terms of the inhibitor's binding to the enzyme and to the enzyme-substrate complex, and its effects on the kinetic constants of the enzyme. In the classic Michaelis-Menten scheme (shown in the "inhibition mechanism schematic" diagram), an enzyme (E) binds to its substrate (S) to form the enzyme–substrate complex ES. Upon catalysis, this complex breaks down to release product P and free enzyme. The inhibitor (I) can bind to either E or ES with thedissociation constant
In chemistry, biochemistry, and pharmacology, a dissociation constant (''K''D) is a specific type of equilibrium constant that measures the propensity of a larger object to separate (dissociate) reversibly into smaller components, as when a complex ...
s ''K''i or ''K''i', respectively.
*Competitive inhibitors can bind to E, but not to ES. Competitive inhibition increases ''K''m (i.e., the inhibitor interferes with substrate binding), but does not affect ''V''max (the inhibitor does not hamper catalysis in ES because it cannot bind to ES).
*Uncompetitive inhibitors bind to ES. Uncompetitive inhibition decreases both ''K''m and ''V''max. The inhibitor affects substrate binding by increasing the enzyme's affinity for the substrate (decreasing ''K''m) as well as hampering catalysis (decreases ''V''max).
*Non-competitive inhibitors have identical affinities for E and ES (''K''i = ''K''i'). Non-competitive inhibition does not change ''K''m (i.e., it does not affect substrate binding) but decreases ''V''max (i.e., inhibitor binding hampers catalysis).
*Mixed-type inhibitors bind to both E and ES, but their affinities for these two forms of the enzyme are different (''K''i ≠ ''K''i'). Thus, mixed-type inhibitors affect substrate binding (increase or decrease ''K''m) and hamper catalysis in the ES complex (decrease ''V''max).
When an enzyme has multiple substrates, inhibitors can show different types of inhibition depending on which substrate is considered. This results from the active site containing two different binding sites within the active site, one for each substrate. For example, an inhibitor might compete with substrate A for the first binding site, but be a non-competitive inhibitor with respect to substrate B in the second binding site.
Traditionally reversible enzyme inhibitors have been classified as competitive, uncompetitive, or non-competitive, according to their effects on ''K''m and ''V''max. These three types of inhibition result respectively from the inhibitor binding only to the enzyme E in the absence of substrate S, to the enzyme–substrate complex ES, or to both. The division of these classes arises from a problem in their derivation and results in the need to use two different binding constants for one binding event. It is further assumed that binding of the inhibitor to the enzyme results in 100% inhibition and fails to consider the possibility of partial inhibition. The common form of the inhibitory term also obscures the relationship between the inhibitor binding to the enzyme and its relationship to any other binding term be it the Michaelis–Menten equation or a dose response curve associated with ligand receptor binding. To demonstrate the relationship the following rearrangement can be made:
:
This rearrangement demonstrates that similar to the Michaelis–Menten equation, the maximal rate of reaction depends on the proportion of the enzyme population interacting with its substrate.
fraction of the enzyme population bound by substrate
:
fraction of the enzyme population bound by inhibitor
:
the effect of the inhibitor is a result of the percent of the enzyme population interacting with inhibitor. The only problem with this equation in its present form is that it assumes absolute inhibition of the enzyme with inhibitor binding, when in fact there can be a wide range of effects anywhere from 100% inhibition of substrate turn over to no inhibition. To account for this the equation can be easily modified to allow for different degrees of inhibition by including a delta ''V''max term.
:
or
:
This term can then define the residual enzymatic activity present when the inhibitor is interacting with individual enzymes in the population. However the inclusion of this term has the added value of allowing for the possibility of activation if the secondary ''V''max term turns out to be higher than the initial term. To account for the possibly of activation as well the notation can then be rewritten replacing the inhibitor "I" with a modifier term (stimulator or inhibitor) denoted here as "X".
:
While this terminology results in a simplified way of dealing with kinetic effects relating to the maximum velocity of the Michaelis–Menten equation, it highlights potential problems with the term used to describe effects relating to the ''K''m. The ''K''m relating to the affinity of the enzyme for the substrate should in most cases relate to potential changes in the binding site of the enzyme which would directly result from enzyme inhibitor interactions. As such a term similar to the delta ''V''max term proposed above to modulate ''V''max should be appropriate in most situations:
:
Dissociation constants
 An enzyme inhibitor is characterised by its
An enzyme inhibitor is characterised by its dissociation constant
In chemistry, biochemistry, and pharmacology, a dissociation constant (''K''D) is a specific type of equilibrium constant that measures the propensity of a larger object to separate (dissociate) reversibly into smaller components, as when a complex ...
''K''i, the concentration at which the inhibitor half occupies the enzyme. In non-competitive inhibition the inhibitor can also bind to the enzyme-substrate complex, and the presence of bound substrate can change the affinity of the inhibitor for the enzyme, resulting in a second dissociation constant ''K''i'. Hence ''K''i and ''K''i' are the dissociation constants of the inhibitor for the enzyme and to the enzyme-substrate complex, respectively. The enzyme-inhibitor constant ''K''i can be measured directly by various methods; one especially accurate method is isothermal titration calorimetry
In chemical thermodynamics, isothermal titration calorimetry (ITC) is a physical technique used to determine the Conjugate variables (thermodynamics), thermodynamic parameters of interactions in Solution (chemistry), solution. ITC is the only tec ...
, in which the inhibitor is titrated into a solution of enzyme and the heat released or absorbed is measured. However, the other dissociation constant ''K''i' is difficult to measure directly, since the enzyme-substrate complex is short-lived and undergoing a chemical reaction to form the product. Hence, ''K''i' is usually measured indirectly, by observing the enzyme activity
Enzyme assays are laboratory methods for measuring enzyme, enzymatic activity. They are vital for the study of enzyme kinetics and enzyme inhibitor, enzyme inhibition.
Enzyme units
The quantity or concentration of an enzyme can be expressed in Mo ...
under various substrate and inhibitor concentrations, and fitting the data via nonlinear regression
In statistics, nonlinear regression is a form of regression analysis in which observational data are modeled by a function which is a nonlinear combination of the model parameters and depends on one or more independent variables. The data are fi ...
to a modified Michaelis–Menten equation.
:
where the modifying factors α and α' are defined by the inhibitor concentration and its two dissociation constants
:
:
Thus, in the presence of the inhibitor, the enzyme's effective ''K''m and ''V''max become (α/α')''K''m and (1/α')''V''max, respectively. However, the modified Michaelis-Menten equation assumes that binding of the inhibitor to the enzyme has reached equilibrium, which may be a very slow process for inhibitors with sub-nanomolar dissociation constants. In these cases the inhibition becomes effectively irreversible, hence it is more practical to treat such tight-binding inhibitors as irreversible (see below
Below may refer to:
*Earth
*Ground (disambiguation)
*Soil
*Floor
* Bottom (disambiguation)
*Less than
*Temperatures below freezing
*Hell or underworld
People with the surname
* Ernst von Below (1863–1955), German World War I general
* Fred Belo ...
).
The effects of different types of reversible enzyme inhibitors on enzymatic activity can be visualised using graphical representations of the Michaelis–Menten equation, such as Lineweaver–Burk, Eadie-Hofstee or Hanes-Woolf plots. An illustration is provided by the three Lineweaver–Burk plots depicted in the ''Lineweaver–Burk diagrams'' figure. In the top diagram the competitive inhibition lines intersect on the ''y''-axis, illustrating that such inhibitors do not affect ''V''max. In the bottom diagram the non-competitive inhibition lines intersect on the ''x''-axis, showing these inhibitors do not affect ''K''m. However, since it can be difficult to estimate ''K''i and ''K''i' accurately from such plots, it is advisable to estimate these constants using more reliable nonlinear regression methods.
Special cases
Partially competitive
The mechanism of partially competitive inhibition is similar to that of non-competitive, except that the EIS complex has catalytic activity, which may be lower or even higher (partially competitive activation) than that of the enzyme–substrate (ES) complex. This inhibition typically displays a lower ''V''max, but an unaffected ''K''m value.Substrate or product
Substrate or product inhibition is where either an enzymes substrate or product also act as an inhibitor. This inhibition may follow the competitive, uncompetitive or mixed patterns. In substrate inhibition there is a progressive decrease in activity at high substrate concentrations, potentially from an enzyme having two competing substrate-binding sites. At low substrate, the high-affinity site is occupied and normal kinetics are followed. However, at higher concentrations, the second inhibitory site becomes occupied, inhibiting the enzyme. Product inhibition (either the enzyme's own product, or a product to an enzyme downstream in its metabolic pathway) is often a regulatory feature in metabolism and can be a form ofnegative feedback
Negative feedback (or balancing feedback) occurs when some function (Mathematics), function of the output of a system, process, or mechanism is feedback, fed back in a manner that tends to reduce the fluctuations in the output, whether caused ...
.
Slow-tight
Slow-tight inhibition occurs when the initial enzyme–inhibitor complex EI undergoesconformational isomerism
In chemistry, rotamers are chemical species that differ from one another primarily due to rotations about one or more single bonds. Various arrangements of atoms in a molecule that differ by rotation about single bonds can also be referred to as ...
(a change in shape) to a second more tightly held complex, EI*, but the overall inhibition process is reversible. This manifests itself as slowly increasing enzyme inhibition. Under these conditions, traditional Michaelis–Menten kinetics give a false value for ''K''i, which is time–dependent. The true value of ''K''i can be obtained through more complex analysis of the on (''k''on) and off (''k''off) rate constants for inhibitor association with kinetics similar to irreversible inhibition.
Multi-substrate analogues
Multi-substrate analogue inhibitors are high affinity selective inhibitors that can be prepared for enzymes that catalyse reactions with more than one substrate by capturing the binding energy of each of those substrate into one molecule. For example, in the formyl transfer reactions of purine biosynthesis, a potent Multi-substrate Adduct Inhibitor (MAI) to glycinamide ribonucleotide (GAR) TFase was prepared synthetically by linking analogues of the GAR substrate and the N-10-formyl tetrahydrofolate cofactor together to produce thioglycinamide ribonucleotide dideazafolate (TGDDF), or enzymatically from the natural GAR substrate to yield GDDF. Here the subnanomolar dissociation constant (KD) of TGDDF was greater than predicted presumably due to entropic advantages gained and/or positive interactions acquired through the atoms linking the components. MAIs have also been observed to be produced in cells by reactions of pro-drugs such asisoniazid
Isoniazid, also known as isonicotinic acid hydrazide (INH), is an antibiotic used for the treatment of tuberculosis. For active tuberculosis, it is often used together with rifampicin, pyrazinamide, and either streptomycin or ethambutol. F ...
or enzyme inhibitor ligands (for example, PTC124) with cellular cofactors such as nicotinamide adenine dinucleotide
Nicotinamide adenine dinucleotide (NAD) is a Cofactor (biochemistry), coenzyme central to metabolism. Found in all living cell (biology), cells, NAD is called a dinucleotide because it consists of two nucleotides joined through their phosphat ...
(NADH) and adenosine triphosphate
Adenosine triphosphate (ATP) is a nucleoside triphosphate that provides energy to drive and support many processes in living cell (biology), cells, such as muscle contraction, nerve impulse propagation, and chemical synthesis. Found in all known ...
(ATP) respectively.
Examples
As enzymes have evolved to bind their substrates tightly, and most reversible inhibitors bind in the active site of enzymes, it is unsurprising that some of these inhibitors are strikingly similar in structure to the substrates of their targets. Inhibitors ofdihydrofolate reductase
Dihydrofolate reductase, or DHFR, is an enzyme that reduces dihydrofolic acid to tetrahydrofolic acid, using NADPH as an electron donor, which can be converted to the kinds of tetrahydrofolate cofactors used in one-carbon transfer chemistry. ...
(DHFR) are prominent examples. Other examples of these substrate mimics are the protease inhibitors
Protease inhibitors (PIs) are medications that act by interfering with protease, enzymes that cleave proteins. Some of the most well known are antiviral drugs widely used to treat HIV/AIDS, hepatitis C and COVID-19. These protease inhibitors pre ...
, a therapeutically effective class of antiretroviral drug
The management of HIV/AIDS normally includes the use of multiple Antiviral drug, antiretroviral drugs as a strategy to control HIV/AIDS, HIV infection. There are several classes of antiretroviral agents that act on different stages of the HIV li ...
s used to treat HIV/AIDS
The HIV, human immunodeficiency virus (HIV) is a retrovirus that attacks the immune system. Without treatment, it can lead to a spectrum of conditions including acquired immunodeficiency syndrome (AIDS). It is a Preventive healthcare, pr ...
. The structure of ritonavir
Ritonavir, sold under the brand name Norvir, is an antiretroviral medication used along with other medications to treat HIV/AIDS. This combination treatment is known as highly active antiretroviral therapy (HAART). Ritonavir is a protease inhi ...
, a peptidomimetic
A peptidomimetic is a small protein-like chain designed to mimic a peptide. They typically arise either from modification of an existing peptide, or by designing similar systems that mimic peptides, such as peptoids and β-peptides. Irrespective ...
(peptide mimic) protease inhibitor containing three peptide bond
In organic chemistry, a peptide bond is an amide type of covalent chemical bond linking two consecutive alpha-amino acids from C1 (carbon number one) of one alpha-amino acid and N2 (nitrogen number two) of another, along a peptide or protein cha ...
s, as shown in the "competitive inhibition" figure above. As this drug resembles the peptide that is the substrate of the HIV protease, it competes with the substrate in the enzyme's active site.
Enzyme inhibitors are often designed to mimic the transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked w ...
or intermediate of an enzyme-catalysed reaction. This ensures that the inhibitor exploits the transition state stabilising effect of the enzyme, resulting in a better binding affinity (lower ''K''i) than substrate-based designs. An example of such a transition state inhibitor is the antiviral drug oseltamivir
Oseltamivir, sold under the brand name Tamiflu among others, is an antiviral medication used to treat and prevent influenza A and influenza B, viruses that cause the flu. Many medical organizations recommend it in people who have complicati ...
; this drug mimics the planar nature of the ring oxonium ion
In chemistry, an oxonium ion is any cation containing an oxygen atom that has three chemical bond, bonds and 1+ formal charge. The simplest oxonium ion is the hydronium ion ().
Alkyloxonium
Hydronium is one of a series of oxonium ions with the fo ...
in the reaction of the viral enzyme neuraminidase
Exo-α-sialidase (, sialidase, neuraminidase; systematic name acetylneuraminyl hydrolase) is a glycoside hydrolase that cleaves the glycosidic linkages of neuraminic acids:
: Hydrolysis of α-(2→3)-, α-(2→6)-, α-(2→8)- glycosidic linkag ...
.
However, not all inhibitors are based on the structures of substrates. For example, the structure of another HIV protease inhibitor tipranavir is not based on a peptide and has no obvious structural similarity to a protein substrate. These non-peptide inhibitors can be more stable than inhibitors containing peptide bonds, because they will not be substrates for peptidase
A protease (also called a peptidase, proteinase, or proteolytic enzyme) is an enzyme that catalyzes proteolysis, breaking down proteins into smaller polypeptides or single amino acids, and spurring the formation of new protein products. They do ...
s and are less likely to be degraded.
In drug design it is important to consider the concentrations of substrates to which the target enzymes are exposed. For example, some protein kinase
A protein kinase is a kinase which selectively modifies other proteins by covalently adding phosphates to them ( phosphorylation) as opposed to kinases which modify lipids, carbohydrates, or other molecules. Phosphorylation usually results in a f ...
inhibitors have chemical structures that are similar to ATP, one of the substrates of these enzymes. However, drugs that are simple competitive inhibitors will have to compete with the high concentrations of ATP in the cell. Protein kinases can also be inhibited by competition at the binding sites where the kinases interact with their substrate proteins, and most proteins are present inside cells at concentrations much lower than the concentration of ATP. As a consequence, if two protein kinase inhibitors both bind in the active site with similar affinity, but only one has to compete with ATP, then the competitive inhibitor at the protein-binding site will inhibit the enzyme more effectively.
Irreversible inhibitors
Types
Irreversible inhibitorscovalent
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
ly bind to an enzyme, and this type of inhibition can therefore not be readily reversed. Irreversible inhibitors often contain reactive functional groups such as nitrogen mustard
Nitrogen mustards (NMs) are cytotoxic organic compounds with the bis(2-chloroethyl)amino ((ClC2H4)2NR) functional group. Although originally produced as chemical warfare agents, they were the first chemotherapeutic agents for treatment of canc ...
s, aldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
s, haloalkane
The haloalkanes (also known as halogenoalkanes or alkyl halides) are alkanes containing one or more halogen substituents of hydrogen atom. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalka ...
s, alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
s, Michael acceptors, phenyl sulfonates, or fluorophosphonates. These electrophilic
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carr ...
groups react with amino acid side chains to form covalent adduct
In chemistry, an adduct (; alternatively, a contraction of "addition product") is a product of a direct addition of two or more distinct molecules, resulting in a single reaction product containing all atoms of all components. The resultant is ...
s. The residues modified are those with side chains containing nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
s such as hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy ...
or sulfhydryl
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl grou ...
groups; these include the amino acids serine
Serine
(symbol Ser or S) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α- amino group (which is in the protonated − form under biological conditions), a carboxyl group (which is in the deprotonated − ...
(that reacts with DFP, see the "DFP reaction" diagram), and also cysteine
Cysteine (; symbol Cys or C) is a semiessential proteinogenic amino acid with the chemical formula, formula . The thiol side chain in cysteine enables the formation of Disulfide, disulfide bonds, and often participates in enzymatic reactions as ...
, threonine
Threonine (symbol Thr or T) is an amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH form when dissolved in water), a carboxyl group (which is in the deprotonated −COO− ...
, or tyrosine
-Tyrosine or tyrosine (symbol Tyr or Y) or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a conditionally essential amino acid with a polar side group. The word "tyrosine" is ...
.
Irreversible inhibition is different from irreversible enzyme inactivation. Irreversible inhibitors are generally specific for one class of enzyme and do not inactivate all proteins; they do not function by destroying protein structure
Protein structure is the three-dimensional arrangement of atoms in an amino acid-chain molecule. Proteins are polymers specifically polypeptides formed from sequences of amino acids, which are the monomers of the polymer. A single amino acid ...
but by specifically altering the active site of their target. For example, extremes of pH or temperature usually cause denaturation of all protein structure, but this is a non-specific effect. Similarly, some non-specific chemical treatments destroy protein structure: for example, heating in concentrated hydrochloric acid
Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungency, pungent smell. It is classified as a acid strength, strong acid. It is ...
will hydrolyse the peptide bond
In organic chemistry, a peptide bond is an amide type of covalent chemical bond linking two consecutive alpha-amino acids from C1 (carbon number one) of one alpha-amino acid and N2 (nitrogen number two) of another, along a peptide or protein cha ...
s holding proteins together, releasing free amino acids.
Irreversible inhibitors display time-dependent inhibition and their potency therefore cannot be characterised by an IC50 value. This is because the amount of active enzyme at a given concentration of irreversible inhibitor will be different depending on how long the inhibitor is pre-incubated with the enzyme. Instead, ''k''obs/ 'I''values are used, where ''k''obs is the observed pseudo-first order rate of inactivation (obtained by plotting the log of % activity versus time) and 'I''is the concentration of inhibitor. The ''k''obs/ 'I''parameter is valid as long as the inhibitor does not saturate binding with the enzyme (in which case ''k''obs = ''k''inact) where ''k''inact is the rate of inactivation.
Measuring
Irreversible inhibitors first form a reversible non-covalent complex with the enzyme (EI or ESI). Subsequently, a chemical reaction occurs between the enzyme and inhibitor to produce the covalently modified "dead-end complex" EI* (an irreversible covalent complex). The rate at which EI* is formed is called the inactivation rate or ''k''inact. Since formation of EI may compete with ES, binding of irreversible inhibitors can be prevented by competition either with substrate or with a second, reversible inhibitor. This protection effect is good evidence of a specific reaction of the irreversible inhibitor with the active site. The binding and inactivation steps of this reaction are investigated by incubating the enzyme with inhibitor and assaying the amount of activity remaining over time. The activity will be decreased in a time-dependent manner, usually followingexponential decay
A quantity is subject to exponential decay if it decreases at a rate proportional to its current value. Symbolically, this process can be expressed by the following differential equation, where is the quantity and (lambda
Lambda (; uppe ...
. Fitting these data to a rate equation
In chemistry, the rate equation (also known as the rate law or empirical differential rate equation) is an Empirical relationship, empirical Differential equation, differential Expression (mathematics), mathematical expression for the reaction rat ...
gives the rate of inactivation at this concentration of inhibitor. This is done at several different concentrations of inhibitor. If a reversible EI complex is involved the inactivation rate will be saturable and fitting this curve will give ''k''inact and ''K''i.
Another method that is widely used in these analyses is mass spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a ''mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is used ...
. Here, accurate measurement of the mass of the unmodified native enzyme and the inactivated enzyme gives the increase in mass caused by reaction with the inhibitor and shows the stoichiometry of the reaction. This is usually done using a MALDI-TOF mass spectrometer. In a complementary technique, peptide mass fingerprinting involves digestion of the native and modified protein with a protease
A protease (also called a peptidase, proteinase, or proteolytic enzyme) is an enzyme that catalysis, catalyzes proteolysis, breaking down proteins into smaller polypeptides or single amino acids, and spurring the formation of new protein products ...
such as trypsin
Trypsin is an enzyme in the first section of the small intestine that starts the digestion of protein molecules by cutting long chains of amino acids into smaller pieces. It is a serine protease from the PA clan superfamily, found in the dig ...
. This will produce a set of peptide
Peptides are short chains of amino acids linked by peptide bonds. A polypeptide is a longer, continuous, unbranched peptide chain. Polypeptides that have a molecular mass of 10,000 Da or more are called proteins. Chains of fewer than twenty am ...
s that can be analysed using a mass spectrometer. The peptide that changes in mass after reaction with the inhibitor will be the one that contains the site of modification.
Slow binding
conformational change
In biochemistry, a conformational change is a change in the shape of a macromolecule, often induced by environmental factors.
A macromolecule is usually flexible and dynamic. Its shape can change in response to changes in its environment or othe ...
as the enzyme "clamps down" around the inhibitor molecule. Examples of slow-binding inhibitors include some important drugs, such methotrexate
Methotrexate, formerly known as amethopterin, is a chemotherapy agent and immunosuppressive drug, immune-system suppressant. It is used to treat cancer, autoimmune diseases, and ectopic pregnancy, ectopic pregnancies. Types of cancers it is u ...
, allopurinol
Allopurinol is a medication used to decrease hyperuricemia, high blood uric acid levels. It is specifically used to prevent gout, prevent specific types of kidney stones and for the high uric acid levels that can occur with chemotherapy. It i ...
, and the activated form of acyclovir
Aciclovir, also known as acyclovir, is an antiviral medication. It is primarily used for the treatment of herpes simplex virus infections, chickenpox, and shingles. Other uses include the prevention of cytomegalovirus infections following tran ...
.
Some examples
 Diisopropylfluorophosphate (DFP) is an example of an irreversible protease inhibitor (see the "DFP reaction" diagram). The enzyme hydrolyses the phosphorus–fluorine bond, but the phosphate residue remains bound to the serine in the
Diisopropylfluorophosphate (DFP) is an example of an irreversible protease inhibitor (see the "DFP reaction" diagram). The enzyme hydrolyses the phosphorus–fluorine bond, but the phosphate residue remains bound to the serine in the active site
In biology and biochemistry, the active site is the region of an enzyme where substrate molecules bind and undergo a chemical reaction. The active site consists of amino acid residues that form temporary bonds with the substrate, the ''binding s ...
, deactivating it. Similarly, DFP also reacts with the active site of acetylcholine esterase
Acetylcholinesterase ( HGNC symbol ACHE; EC 3.1.1.7; systematic name acetylcholine acetylhydrolase), also known as AChE, AChase or acetylhydrolase, is the primary cholinesterase in the body. It is an enzyme that catalyzes the breakdown of ac ...
in the synapses
In the nervous system, a synapse is a structure that allows a neuron (or nerve cell) to pass an electrical or chemical signal to another neuron or a target effector cell. Synapses can be classified as either chemical or electrical, depending o ...
of neurons, and consequently is a potent neurotoxin, with a lethal dose of less than 100mg.
Suicide inhibition is an unusual type of irreversible inhibition where the enzyme converts the inhibitor into a reactive form in its active site. An example is the inhibitor of polyamine
A polyamine is an organic compound having two or more amino groups. Alkyl polyamines occur naturally, but some are synthetic. Alkylpolyamines are colorless, hygroscopic, and water soluble. Near neutral pH, they exist as the ammonium derivatives. ...
biosynthesis, α-difluoromethylornithine (DFMO), which is an analogue of the amino acid ornithine
Ornithine is a non-proteinogenic α-amino acid that plays a role in the urea cycle. It is not incorporated into proteins during translation. Ornithine is abnormally accumulated in the body in ornithine transcarbamylase deficiency, a disorder of th ...
, and is used to treat African trypanosomiasis
African trypanosomiasis is an insect-borne parasitic infection of humans and other animals.
Human African trypanosomiasis (HAT), also known as African sleeping sickness or simply sleeping sickness, is caused by the species ''Trypanosoma bru ...
(sleeping sickness). Ornithine decarboxylase
The enzyme ornithine decarboxylase (, ODC) catalyzes the decarboxylation of ornithine (a product of the urea cycle) to form putrescine. This reaction is the committed step in polyamine synthesis. In humans, this protein has 461 amino acids ...
can catalyse the decarboxylation of DFMO instead of ornithine (see the "DFMO inhibitor mechanism" diagram). However, this decarboxylation reaction is followed by the elimination of a fluorine atom, which converts this catalytic intermediate into a conjugated imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bon ...
, a highly electrophilic species. This reactive form of DFMO then reacts with either a cysteine or lysine residue in the active site to irreversibly inactivate the enzyme.
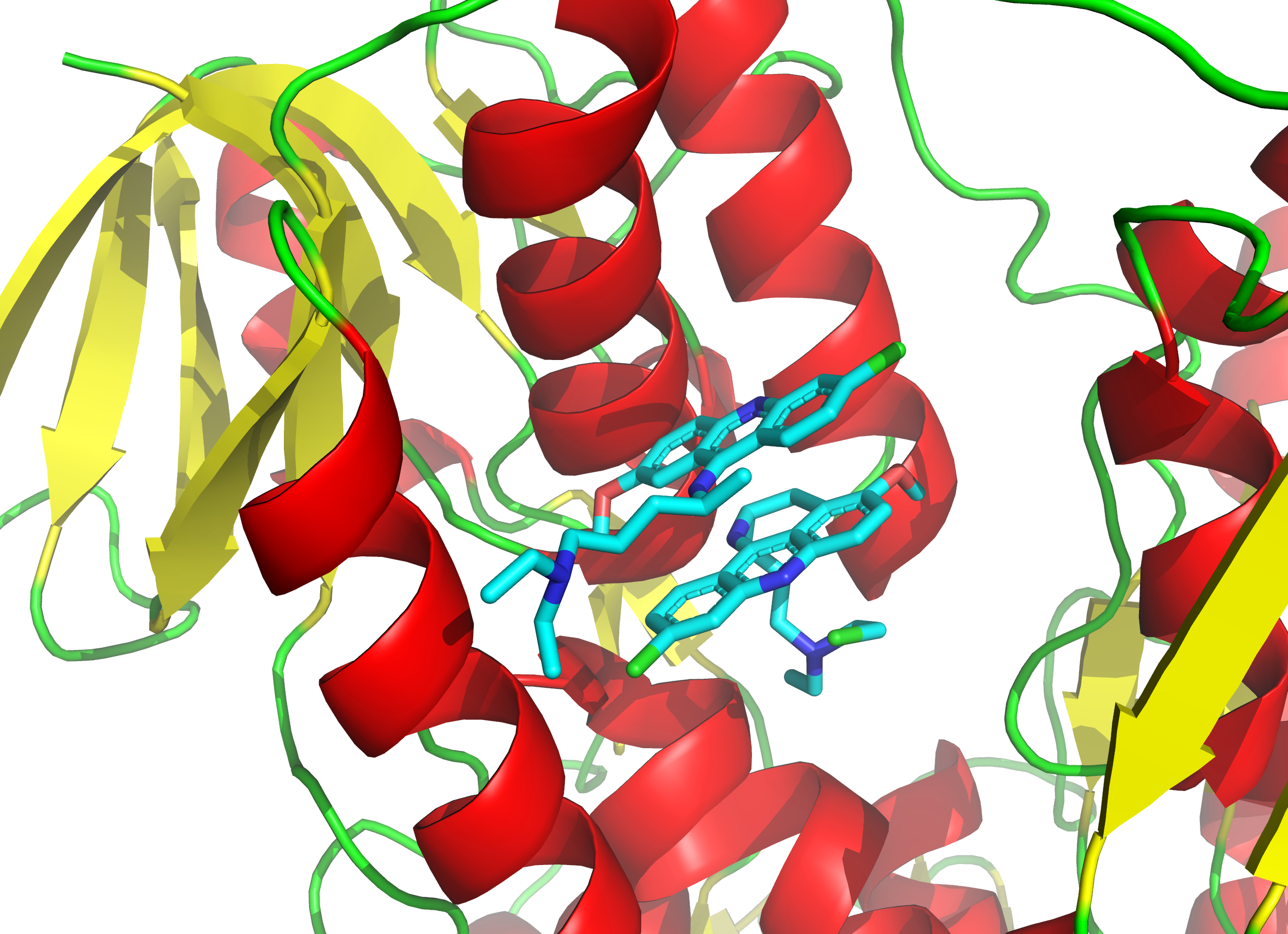
Since irreversible inhibition often involves the initial formation of a non-covalent enzyme inhibitor (EI) complex, it is sometimes possible for an inhibitor to bind to an enzyme in more than one way. For example, in the figure showing trypanothione reductase from the human protozoan parasite ''Trypanosoma cruzi
''Trypanosoma cruzi'' is a species of parasitic euglenoids. Among the protozoa, the trypanosomes characteristically bore tissue in another organism and feed on blood (primarily) and also lymph. This behaviour causes disease or the likelihood ...
'', two molecules of an inhibitor called ''quinacrine mustard'' are bound in its active site. The top molecule is bound reversibly, but the lower one is bound covalently as it has reacted with an amino acid residue through its nitrogen mustard
Nitrogen mustards (NMs) are cytotoxic organic compounds with the bis(2-chloroethyl)amino ((ClC2H4)2NR) functional group. Although originally produced as chemical warfare agents, they were the first chemotherapeutic agents for treatment of canc ...
group.
Applications
Enzyme inhibitors are found in nature and also produced artificially in the laboratory. Naturally occurring enzyme inhibitors regulate manymetabolic
Metabolism (, from ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run cellular processes; the ...
processes and are essential for life. In addition, naturally produced poison
A poison is any chemical substance that is harmful or lethal to living organisms. The term is used in a wide range of scientific fields and industries, where it is often specifically defined. It may also be applied colloquially or figurati ...
s are often enzyme inhibitors that have evolved for use as toxic agents against predators, prey, and competing organisms. These natural toxins include some of the most poisonous substances known. Artificial inhibitors are often used as drugs, but can also be insecticide
Insecticides are pesticides used to kill insects. They include ovicides and larvicides used against insect eggs and larvae, respectively. The major use of insecticides is in agriculture, but they are also used in home and garden settings, i ...
s such as malathion
Malathion is an organophosphate insecticide which acts as an acetylcholinesterase inhibitor. In the USSR, it was known as carbophos, in New Zealand and Australia as maldison and in South Africa as mercaptothion. The compound's name is presumably ...
, herbicide
Herbicides (, ), also commonly known as weed killers, are substances used to control undesired plants, also known as weeds.EPA. February 201Pesticides Industry. Sales and Usage 2006 and 2007: Market Estimates. Summary in press releasMain page f ...
s such as glyphosate
Glyphosate (IUPAC name: ''N''-(phosphonomethyl)glycine) is a broad-spectrum systemic herbicide and crop desiccant. It is an organophosphorus compound, specifically a phosphonate, which acts by EPSP inhibitor, inhibiting the plant enzyme 5-en ...
, or disinfectant
A disinfectant is a chemical substance or compound used to inactivate or destroy microorganisms on inert surfaces. Disinfection does not necessarily kill all microorganisms, especially resistant bacterial spores; it is less effective than ...
s such as triclosan
Triclosan (sometimes abbreviated as TCS) is an antibacterial and antifungal agent present in some consumer products, including toothpaste, soaps, detergents, toys, and surgical cleaning treatments. It is similar in its uses and mechanism of act ...
. Other artificial enzyme inhibitors block acetylcholinesterase
Acetylcholinesterase (HUGO Gene Nomenclature Committee, HGNC symbol ACHE; EC 3.1.1.7; systematic name acetylcholine acetylhydrolase), also known as AChE, AChase or acetylhydrolase, is the primary cholinesterase in the body. It is an enzyme th ...
, an enzyme which breaks down acetylcholine
Acetylcholine (ACh) is an organic compound that functions in the brain and body of many types of animals (including humans) as a neurotransmitter. Its name is derived from its chemical structure: it is an ester of acetic acid and choline. Par ...
, and are used as nerve agent
Nerve agents, sometimes also called nerve gases, are a class of organic chemistry, organic chemicals that disrupt the mechanisms by which nerves transfer messages to organs. The disruption is caused by the blocking of acetylcholinesterase (ACh ...
s in chemical warfare.
Metabolic regulation
Enzyme inhibition is a common feature ofmetabolic pathway
In biochemistry, a metabolic pathway is a linked series of chemical reactions occurring within a cell (biology), cell. The reactants, products, and Metabolic intermediate, intermediates of an enzymatic reaction are known as metabolites, which are ...
control in cells. Metabolic flux through a pathway is often regulated by a pathway's metabolites
In biochemistry, a metabolite is an intermediate or end product of metabolism.
The term is usually used for small molecules. Metabolites have various functions, including fuel, structure, signaling, stimulatory and inhibitory effects on enzymes, c ...
acting as inhibitors and enhancers for the enzymes in that same pathway. The glycolytic pathway is a classic example. This catabolic
Catabolism () is the set of metabolic pathways that breaks down molecules into smaller units that are either oxidized to release energy or used in other anabolic reactions. Catabolism breaks down large molecules (such as polysaccharides, lipi ...
pathway consumes glucose
Glucose is a sugar with the Chemical formula#Molecular formula, molecular formula , which is often abbreviated as Glc. It is overall the most abundant monosaccharide, a subcategory of carbohydrates. It is mainly made by plants and most algae d ...
and produces ATP, NADH
Nicotinamide adenine dinucleotide (NAD) is a coenzyme central to metabolism. Found in all living cells, NAD is called a dinucleotide because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an ade ...
and pyruvate
Pyruvic acid (CH3COCOOH) is the simplest of the alpha-keto acids, with a carboxylic acid and a ketone functional group. Pyruvate, the conjugate base, CH3COCOO−, is an intermediate in several metabolic pathways throughout the cell.
Pyruvic ...
. A key step for the regulation of glycolysis is an early reaction in the pathway catalysed by phosphofructokinase1 (PFK1). When ATP levels rise, ATP binds an allosteric site in PFK1 to decrease the rate of the enzyme reaction; glycolysis is inhibited and ATP production falls. This negative feedback
Negative feedback (or balancing feedback) occurs when some function (Mathematics), function of the output of a system, process, or mechanism is feedback, fed back in a manner that tends to reduce the fluctuations in the output, whether caused ...
control helps maintain a steady concentration of ATP in the cell. However, metabolic pathways are not just regulated through inhibition since enzyme activation is equally important. With respect to PFK1, fructose 2,6-bisphosphate
Fructose 2,6-bisphosphate, abbreviated Fru-2,6-''P''2, is a metabolite that allosterically affects the activity of the enzymes phosphofructokinase 1 (PFK-1) and fructose 1,6-bisphosphatase (FBPase-1) to regulate glycolysis and gluconeogenesis. ...
and ADP are examples of metabolites that are allosteric activators.
Physiological enzyme inhibition can also be produced by specific protein inhibitors. This mechanism occurs in the pancreas
The pancreas (plural pancreases, or pancreata) is an Organ (anatomy), organ of the Digestion, digestive system and endocrine system of vertebrates. In humans, it is located in the abdominal cavity, abdomen behind the stomach and functions as a ...
, which synthesises many digestive precursor enzymes known as zymogen
In biochemistry, a zymogen (), also called a proenzyme (), is an inactive precursor of an enzyme. A zymogen requires a biochemical change (such as a hydrolysis reaction revealing the active site, or changing the configuration to reveal the activ ...
s. Many of these are activated by the trypsin
Trypsin is an enzyme in the first section of the small intestine that starts the digestion of protein molecules by cutting long chains of amino acids into smaller pieces. It is a serine protease from the PA clan superfamily, found in the dig ...
protease, so it is important to inhibit the activity of trypsin in the pancreas to prevent the organ from digesting itself. One way in which the activity of trypsin is controlled is the production of a specific and potent trypsin inhibitor
A trypsin inhibitor (TI) is a protein and a type of serine protease inhibitor ( serpin) that reduces the biological activity of trypsin by controlling the activation and catalytic reactions of proteins. Trypsin is an enzyme involved in the breakdow ...
protein in the pancreas. This inhibitor binds tightly to trypsin, preventing the trypsin activity that would otherwise be detrimental to the organ. Although the trypsin inhibitor is a protein, it avoids being hydrolysed as a substrate by the protease by excluding water from trypsin's active site and destabilising the transition state. Other examples of physiological enzyme inhibitor proteins include the barstar inhibitor of the bacterial ribonuclease barnase.
Natural poisons
 Animals and plants have evolved to synthesise a vast array of poisonous products including
Animals and plants have evolved to synthesise a vast array of poisonous products including secondary metabolite
Secondary metabolites, also called ''specialised metabolites'', ''secondary products'', or ''natural products'', are organic compounds produced by any lifeform, e.g. bacteria, archaea, fungi, animals, or plants, which are not directly involved ...
s, peptides and proteins that can act as inhibitors. Natural toxins are usually small organic molecules and are so diverse that there are probably natural inhibitors for most metabolic processes. The metabolic processes targeted by natural poisons encompass more than enzymes in metabolic pathways and can also include the inhibition of receptor, channel and structural protein functions in a cell. For example, paclitaxel
Paclitaxel, sold under the brand name Taxol among others, is a chemotherapy medication used to treat ovarian cancer, esophageal cancer, breast cancer, lung cancer, Kaposi's sarcoma, cervical cancer, and pancreatic cancer. It is administered b ...
(taxol), an organic molecule found in the Pacific yew tree, binds tightly to tubulin
Tubulin in molecular biology can refer either to the tubulin protein superfamily of globular proteins, or one of the member proteins of that superfamily. α- and β-tubulins polymerize into microtubules, a major component of the eukaryotic cytosk ...
dimers and inhibits their assembly into microtubule
Microtubules are polymers of tubulin that form part of the cytoskeleton and provide structure and shape to eukaryotic cells. Microtubules can be as long as 50 micrometres, as wide as 23 to 27 nanometer, nm and have an inner diameter bet ...
s in the cytoskeleton
The cytoskeleton is a complex, dynamic network of interlinking protein filaments present in the cytoplasm of all cells, including those of bacteria and archaea. In eukaryotes, it extends from the cell nucleus to the cell membrane and is compos ...
.
Many natural poisons act as neurotoxin
Neurotoxins are toxins that are destructive to nervous tissue, nerve tissue (causing neurotoxicity). Neurotoxins are an extensive class of exogenous chemical neurological insult (medical), insultsSpencer 2000 that can adversely affect function ...
s that can cause paralysis
Paralysis (: paralyses; also known as plegia) is a loss of Motor skill, motor function in one or more Skeletal muscle, muscles. Paralysis can also be accompanied by a loss of feeling (sensory loss) in the affected area if there is sensory d ...
leading to death and function for defence against predators or in hunting and capturing prey. Some of these natural inhibitors, despite their toxic attributes, are valuable for therapeutic uses at lower doses. An example of a neurotoxin are the glycoalkaloid
Glycoalkaloids are a family of chemical compounds derived from alkaloids to which sugar groups are appended. Several are potentially toxic, most notably the poisons commonly found in the plant species '' Solanum dulcamara'' (bittersweet nightshad ...
s, from the plant species in the family Solanaceae
Solanaceae (), commonly known as the nightshades, is a family of flowering plants in the order Solanales. It contains approximately 2,700 species, several of which are used as agricultural crops, medicinal plants, and ornamental plants. Many me ...
(includes potato
The potato () is a starchy tuberous vegetable native to the Americas that is consumed as a staple food in many parts of the world. Potatoes are underground stem tubers of the plant ''Solanum tuberosum'', a perennial in the nightshade famil ...
, tomato
The tomato (, ), ''Solanum lycopersicum'', is a plant whose fruit is an edible Berry (botany), berry that is eaten as a vegetable. The tomato is a member of the nightshade family that includes tobacco, potato, and chili peppers. It originate ...
and eggplant
Eggplant (American English, US, Canadian English, CA, Australian English, AU, Philippine English, PH), aubergine (British English, UK, Hiberno English, IE, New Zealand English, NZ), brinjal (Indian English, IN, Singapore English, SG, Malays ...
), that are acetylcholinesterase inhibitor
Acetylcholinesterase inhibitors (AChEIs) also often called cholinesterase inhibitors, inhibit the enzyme acetylcholinesterase from breaking down the neurotransmitter acetylcholine into choline and acetate, thereby increasing both the level an ...
s. Inhibition of this enzyme causes an uncontrolled increase in the acetylcholine neurotransmitter, muscular paralysis and then death. Neurotoxicity
Neurotoxicity is a form of toxicity in which a biological, chemical, or physical agent produces an adverse effect on the structure or function of the central and/or peripheral nervous system. It occurs when exposure to a substance – specifical ...
can also result from the inhibition of receptors; for example, atropine
Atropine is a tropane alkaloid and anticholinergic medication used to treat certain types of nerve agent and pesticide poisonings as well as some types of slow heart rate, and to decrease saliva production during surgery. It is typically give ...
from deadly nightshade (''Atropa belladonna
''Atropa bella-donna'', commonly known as deadly nightshade or belladonna, is a toxic perennial herbaceous plant in the nightshade family Solanaceae, which also includes tomatoes, potatoes and eggplant. It is native to Europe and Western Asia, i ...
'') that functions as a competitive antagonist
A receptor antagonist is a type of receptor ligand or drug that blocks or dampens a biological response by binding to and blocking a receptor rather than activating it like an agonist. Antagonist drugs interfere in the natural operation of recep ...
of the muscarinic acetylcholine receptors.
Although many natural toxins are secondary metabolites, these poisons also include peptides and proteins. An example of a toxic peptide is alpha-amanitin, which is found in relatives of the death cap
''Amanita phalloides'' ( ), commonly known as the death cap, is a deadly poisonous basidiomycete fungus and mushroom, one of many in the genus ''Amanita''. Originating in Europe but later introduced to other parts of the world since the late ...
mushroom. This is a potent enzyme inhibitor, in this case preventing the RNA polymerase II enzyme from transcribing DNA. The algal toxin microcystin is also a peptide and is an inhibitor of protein phosphatases. This toxin can contaminate water supplies after algal bloom
An algal bloom or algae bloom is a rapid increase or accumulation in the population of algae in fresh water or marine water systems. It is often recognized by the discoloration in the water from the algae's pigments. The term ''algae'' encompass ...
s and is a known carcinogen that can also cause acute liver haemorrhage and death at higher doses.
Proteins can also be natural poisons or antinutrient
Antinutrients are natural or synthetic compounds that interfere with the absorption of nutrients. Nutrition studies focus on antinutrients commonly found in food sources and beverages. Antinutrients may take the form of drugs, chemicals that natur ...
s, such as the trypsin inhibitor
A trypsin inhibitor (TI) is a protein and a type of serine protease inhibitor ( serpin) that reduces the biological activity of trypsin by controlling the activation and catalytic reactions of proteins. Trypsin is an enzyme involved in the breakdow ...
s (discussed in the "metabolic regulation" section above) that are found in some legume
Legumes are plants in the pea family Fabaceae (or Leguminosae), or the fruit or seeds of such plants. When used as a dry grain for human consumption, the seeds are also called pulses. Legumes are grown agriculturally, primarily for human consum ...
s. A less common class of toxins are toxic enzymes: these act as irreversible inhibitors of their target enzymes and work by chemically modifying their substrate enzymes. An example is ricin
Ricin ( ) is a lectin (a carbohydrate-binding protein) and a highly potent toxin produced in the seeds of the castor oil plant, ''Ricinus communis''. The median lethal dose (LD50) of ricin for mice is around 22 micrograms per kilogram of body ...
, an extremely potent protein toxin found in castor oil beans. This enzyme is a glycosidase that inactivates ribosomes. Since ricin is a catalytic irreversible inhibitor, this allows just a single molecule of ricin to kill a cell.
Drugs
aspirin
Aspirin () is the genericized trademark for acetylsalicylic acid (ASA), a nonsteroidal anti-inflammatory drug (NSAID) used to reduce pain, fever, and inflammation, and as an antithrombotic. Specific inflammatory conditions that aspirin is ...
is a widely used drug that acts as a suicide inhibitor of the cyclooxygenase
Cyclooxygenase (COX), officially known as prostaglandin-endoperoxide synthase (PTGS), is an enzyme (specifically, a family of isozymes, ) that is responsible for biosynthesis of prostanoids, including thromboxane and prostaglandins such a ...
enzyme. This inhibition in turn suppresses the production of proinflammatory prostaglandin
Prostaglandins (PG) are a group of physiology, physiologically active lipid compounds called eicosanoids that have diverse hormone-like effects in animals. Prostaglandins have been found in almost every Tissue (biology), tissue in humans and ot ...
s and thus aspirin may be used to reduce pain, fever, and inflammation.
an estimated 29% of approved drugs are enzyme inhibitors of which approximately one-fifth are kinase
In biochemistry, a kinase () is an enzyme that catalyzes the transfer of phosphate groups from high-energy, phosphate-donating molecules to specific substrates. This process is known as phosphorylation, where the high-energy ATP molecule don ...
inhibitors. A notable class of kinase drug targets is the receptor tyrosine kinase
Receptor tyrosine kinases (RTKs) are the high-affinity cell surface receptors for many polypeptide growth factors, cytokines, and hormones. Of the 90 unique tyrosine kinase genes identified in the human genome, 58 encode receptor tyrosine kinas ...
s which are essential enzymes that regulate cell growth
Cell most often refers to:
* Cell (biology), the functional basic unit of life
* Cellphone, a phone connected to a cellular network
* Clandestine cell, a penetration-resistant form of a secret or outlawed organization
* Electrochemical cell, a de ...
; their over-activation may result in cancer. Hence kinase inhibitors such as imatinib
Imatinib, sold under the brand names Gleevec and Glivec (both marketed worldwide by Novartis) among others, is an oral targeted therapy medication used to treat cancer. Imatinib is a small molecule inhibitor targeting multiple tyrosine kinases ...
are frequently used to treat malignancies. Janus kinase
Janus kinase (JAK) is a family of intracellular, non-receptor tyrosine kinases that transduce cytokine-mediated signals via the JAK-STAT pathway. They were initially named "just another kinase" 1 and 2 (since they were just two of many discoverie ...
s are another notable example of drug enzyme targets. Inhibitors of Janus kinases block the production of inflammatory cytokine
An inflammatory cytokine or proinflammatory cytokine is a type of signaling molecule (a cytokine) that is secreted from immune cells like helper T cells (Th) and macrophages, and certain other cell types that promote inflammation. They include int ...
s and hence these inhibitors are used to treat a variety of inflammatory disease
Inflammation (from ) is part of the biological response of body tissues to harmful stimuli, such as pathogens, damaged cells, or Irritation, irritants. The five cardinal signs are heat, pain, redness, swelling, and Functio laesa, loss of funct ...
s in including arthritis
Arthritis is a general medical term used to describe a disorder that affects joints. Symptoms generally include joint pain and stiffness. Other symptoms may include redness, warmth, Joint effusion, swelling, and decreased range of motion of ...
, asthma
Asthma is a common long-term inflammatory disease of the airways of the lungs. It is characterized by variable and recurring symptoms, reversible airflow obstruction, and easily triggered bronchospasms. Symptoms include episodes of wh ...
, and Crohn's disease
Crohn's disease is a type of inflammatory bowel disease (IBD) that may affect any segment of the gastrointestinal tract. Symptoms often include abdominal pain, diarrhea, fever, abdominal distension, and weight loss. Complications outside of the ...
.
An example of the structural similarity of some inhibitors to the substrates of the enzymes they target is seen in the figure comparing the drug methotrexate
Methotrexate, formerly known as amethopterin, is a chemotherapy agent and immunosuppressive drug, immune-system suppressant. It is used to treat cancer, autoimmune diseases, and ectopic pregnancy, ectopic pregnancies. Types of cancers it is u ...
to folic acid
Folate, also known as vitamin B9 and folacin, is one of the B vitamins. Manufactured folic acid, which is converted into folate by the body, is used as a dietary supplement and in food fortification as it is more stable during processing and ...
. Folic acid is the oxidised form of the substrate of dihydrofolate reductase
Dihydrofolate reductase, or DHFR, is an enzyme that reduces dihydrofolic acid to tetrahydrofolic acid, using NADPH as an electron donor, which can be converted to the kinds of tetrahydrofolate cofactors used in one-carbon transfer chemistry. ...
, an enzyme that is potently inhibited by methotrexate. Methotrexate blocks the action of dihydrofolate reductase and thereby halts thymidine
Thymidine (nucleoside#List of nucleosides and corresponding nucleobases, symbol dT or dThd), also known as deoxythymidine, deoxyribosylthymine, or thymine deoxyriboside, is a pyrimidine nucleoside, deoxynucleoside. Deoxythymidine is the DNA nuc ...
biosynthesis. This block of nucleotide
Nucleotides are Organic compound, organic molecules composed of a nitrogenous base, a pentose sugar and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both o ...
biosynthesis is selectively toxic to rapidly growing cells, therefore methotrexate is often used in cancer chemotherapy.
A common treatment for erectile dysfunction
Erectile dysfunction (ED), also referred to as impotence, is a form of sexual dysfunction in males characterized by the persistent or recurring inability to achieve or maintain a Human penis, penile erection with sufficient rigidity and durat ...
is sildenafil
Sildenafil, sold under the brand name Viagra among others, is a medication used to treat erectile dysfunction and pulmonary hypertension, pulmonary arterial hypertension. It is also sometimes used off-label for the treatment of certain sym ...
(Viagra). This compound is a potent inhibitor of cGMP specific phosphodiesterase type 5, the enzyme that degrades the signalling molecule cyclic guanosine monophosphate
Cyclic guanosine monophosphate (cGMP) is a cyclic nucleotide derived from guanosine triphosphate (GTP). cGMP acts as a second messenger much like cyclic AMP. Its most likely mechanism of action is activation of intracellular protein kinases in ...
. This signalling molecule triggers smooth muscle relaxation and allows blood flow into the corpus cavernosum, which causes an erection. Since the drug decreases the activity of the enzyme that halts the signal, it makes this signal last for a longer period of time.
Antibiotics
 Drugs are also used to inhibit enzymes needed for the survival of pathogens. For example, bacteria are surrounded by a thick
Drugs are also used to inhibit enzymes needed for the survival of pathogens. For example, bacteria are surrounded by a thick cell wall
A cell wall is a structural layer that surrounds some Cell type, cell types, found immediately outside the cell membrane. It can be tough, flexible, and sometimes rigid. Primarily, it provides the cell with structural support, shape, protection, ...
made of a net-like polymer called peptidoglycan
Peptidoglycan or murein is a unique large macromolecule, a polysaccharide, consisting of sugars and amino acids that forms a mesh-like layer (sacculus) that surrounds the bacterial cytoplasmic membrane. The sugar component consists of alternating ...
. Many antibiotic
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting pathogenic bacteria, bacterial infections, and antibiotic medications are widely used in the therapy ...
s such as penicillin
Penicillins (P, PCN or PEN) are a group of beta-lactam antibiotic, β-lactam antibiotics originally obtained from ''Penicillium'' Mold (fungus), moulds, principally ''Penicillium chrysogenum, P. chrysogenum'' and ''Penicillium rubens, P. ru ...
and vancomycin
Vancomycin is a glycopeptide antibiotic medication used to treat certain bacterial infections. It is administered intravenously ( injection into a vein) to treat complicated skin infections, bloodstream infections, endocarditis, bone an ...
inhibit the enzymes that produce and then cross-link the strands of this polymer together. This causes the cell wall to lose strength and the bacteria to burst. In the figure, a molecule of penicillin (shown in a ball-and-stick form) is shown bound to its target, the transpeptidase from the bacteria ''Streptomyces'' R61 (the protein is shown as a ribbon diagram
Ribbon diagrams, also known as Richardson diagrams, are three-dimensional space, 3D schematic representations of protein structure and are one of the most common methods of protein depiction used today. The ribbon depicts the general course and o ...
).
Antibiotic drug design
Drug design, often referred to as rational drug design or simply rational design, is the invention, inventive process of finding new medications based on the knowledge of a biological target. The drug is most commonly an organic compound, organi ...
is facilitated when an enzyme that is essential to the pathogen's survival is absent or very different in humans. Humans do not make peptidoglycan, therefore antibiotics that inhibit this process are selectively toxic to bacteria. Selective toxicity is also produced in antibiotics by exploiting differences in the structure of the ribosome
Ribosomes () are molecular machine, macromolecular machines, found within all cell (biology), cells, that perform Translation (biology), biological protein synthesis (messenger RNA translation). Ribosomes link amino acids together in the order s ...
s in bacteria, or how they make fatty acid
In chemistry, in particular in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated and unsaturated compounds#Organic chemistry, saturated or unsaturated. Most naturally occurring fatty acids have an ...
s.
Antivirals
Drugs that inhibit enzymes needed for the replication of viruses are effective in treating viral infections.Antiviral drug
Antiviral drugs are a class of medication used for treating viral infections. Most antivirals target specific viruses, while a broad-spectrum antiviral is effective against a wide range of viruses. Antiviral drugs are a class of antimicrobials ...
s include protease inhibitors
Protease inhibitors (PIs) are medications that act by interfering with protease, enzymes that cleave proteins. Some of the most well known are antiviral drugs widely used to treat HIV/AIDS, hepatitis C and COVID-19. These protease inhibitors pre ...
used to treat HIV/AIDS
The HIV, human immunodeficiency virus (HIV) is a retrovirus that attacks the immune system. Without treatment, it can lead to a spectrum of conditions including acquired immunodeficiency syndrome (AIDS). It is a Preventive healthcare, pr ...
and Hepatitis C, reverse-transcriptase inhibitor
Reverse-transcriptase inhibitors (RTIs) are a class of antiretroviral drugs used to treat HIV infection or AIDS, and in some cases hepatitis B. RTIs inhibit activity of reverse transcriptase, a viral DNA polymerase that is required for replicatio ...
s targeting HIV/AIDS, neuraminidase inhibitors targeting influenza
Influenza, commonly known as the flu, is an infectious disease caused by influenza viruses. Symptoms range from mild to severe and often include fever, runny nose, sore throat, muscle pain, headache, coughing, and fatigue. These sympto ...
, and terminase inhibitors targeting human cytomegalovirus
Human cytomegalovirus (HCMV), also called human herpesvirus 5 (HHV-5), is a species of virus in the genus ''Cytomegalovirus'', which in turn is a member of the viral family known as ''Herpesviridae'' or herpesviruses. It is also commonly call ...
.
Pesticides
Manypesticide
Pesticides are substances that are used to control pests. They include herbicides, insecticides, nematicides, fungicides, and many others (see table). The most common of these are herbicides, which account for approximately 50% of all p ...
s are enzyme inhibitors. Acetylcholinesterase
Acetylcholinesterase (HUGO Gene Nomenclature Committee, HGNC symbol ACHE; EC 3.1.1.7; systematic name acetylcholine acetylhydrolase), also known as AChE, AChase or acetylhydrolase, is the primary cholinesterase in the body. It is an enzyme th ...
(AChE) is an enzyme found in animals, from insects to humans. It is essential to nerve cell function through its mechanism of breaking down the neurotransmitter acetylcholine
Acetylcholine (ACh) is an organic compound that functions in the brain and body of many types of animals (including humans) as a neurotransmitter. Its name is derived from its chemical structure: it is an ester of acetic acid and choline. Par ...
into its constituents, acetate
An acetate is a salt formed by the combination of acetic acid with a base (e.g. alkaline, earthy, metallic, nonmetallic, or radical base). "Acetate" also describes the conjugate base or ion (specifically, the negatively charged ion called ...
and choline
Choline is a cation with the chemical formula . Choline forms various Salt (chemistry), salts, such as choline chloride and choline bitartrate. An essential nutrient for animals, it is a structural component of phospholipids and cell membrane ...
. This is somewhat unusual among neurotransmitters as most, including serotonin
Serotonin (), also known as 5-hydroxytryptamine (5-HT), is a monoamine neurotransmitter with a wide range of functions in both the central nervous system (CNS) and also peripheral tissues. It is involved in mood, cognition, reward, learning, ...
, dopamine
Dopamine (DA, a contraction of 3,4-dihydroxyphenethylamine) is a neuromodulatory molecule that plays several important roles in cells. It is an organic chemical of the catecholamine and phenethylamine families. It is an amine synthesized ...
, and norepinephrine
Norepinephrine (NE), also called noradrenaline (NA) or noradrenalin, is an organic compound, organic chemical in the catecholamine family that functions in the brain and human body, body as a hormone, neurotransmitter and neuromodulator. The ...
, are absorbed from the synaptic cleft
Chemical synapses are biological junctions through which neurons' signals can be sent to each other and to non-neuronal cells such as those in neuromuscular junction, muscles or glands. Chemical synapses allow neurons to form biological neural ...
rather than cleaved. A large number of AChE inhibitors are used in both medicine and agriculture. Reversible competitive inhibitors, such as edrophonium, physostigmine
Physostigmine (also known as eserine from ''éséré'', the West African name for the Calabar bean) is a highly toxic parasympathomimetic alkaloid, specifically, a reversible cholinesterase inhibitor. It occurs naturally in the Calabar bean and ...
, and neostigmine
Neostigmine, sold under the brand name Bloxiverz, among others, is a medication used to treat myasthenia gravis, Ogilvie syndrome, and urinary retention without the presence of a blockage. It is also used in anaesthesia to end the effects of n ...
, are used in the treatment of myasthenia gravis
Myasthenia gravis (MG) is a long-term neuromuscular junction disease that leads to varying degrees of skeletal muscle weakness. The most commonly affected muscles are those of the eyes, face, and swallowing. It can result in double vision, ...
and in anaesthesia to reverse muscle blockade. The carbamate
In organic chemistry, a carbamate is a category of organic compounds with the general Chemical formula, formula and Chemical structure, structure , which are formally Derivative (chemistry), derived from carbamic acid (). The term includes orga ...
pesticides are also examples of reversible AChE inhibitors. The organophosphate
In organic chemistry, organophosphates (also known as phosphate esters, or OPEs) are a class of organophosphorus compounds with the general structure , a central phosphate molecule with alkyl or aromatic substituents. They can be considered ...
pesticides such as malathion
Malathion is an organophosphate insecticide which acts as an acetylcholinesterase inhibitor. In the USSR, it was known as carbophos, in New Zealand and Australia as maldison and in South Africa as mercaptothion. The compound's name is presumably ...
, parathion
Parathion, also called parathion-ethyl or diethyl parathion, is an organophosphate insecticide and acaricide. It was originally developed by IG Farben in the 1940s. It is highly toxic to non-target organisms, including humans, so its use has been ...
, and chlorpyrifos
Chlorpyrifos (CPS), also known as chlorpyrifos ethyl, is an organophosphate pesticide that has been used on crops, animals, in buildings, and in other settings, to kill several pests, including insects and worms. It acts on the nervous systems ...
irreversibly inhibit acetylcholinesterase.
Herbicides
The herbicideglyphosate
Glyphosate (IUPAC name: ''N''-(phosphonomethyl)glycine) is a broad-spectrum systemic herbicide and crop desiccant. It is an organophosphorus compound, specifically a phosphonate, which acts by EPSP inhibitor, inhibiting the plant enzyme 5-en ...
is an inhibitor of 3-phosphoshikimate 1-carboxyvinyltransferase, other herbicides, such as the sulfonylurea
Sulfonylureas or sulphonylureas are a class of organic compounds used in medicine and agriculture. The functional group consists of a sulfonyl group (-S(=O)2) with its sulphur atom bonded to a nitrogen atom of a ureylene group (N,N-dehydrourea ...
s inhibit the enzyme acetolactate synthase
The acetolactate synthase (ALS) enzyme (also known as acetohydroxy acid or acetohydroxyacid synthase, abbr. AHAS) is a protein found in plants and micro-organisms. ALS catalyzes the first step in the synthesis of the branched-chain amino acids ...
. Both enzymes are needed for plants to make branched-chain amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 a ...
s. Many other enzymes are inhibited by herbicides, including enzymes needed for the biosynthesis of lipid
Lipids are a broad group of organic compounds which include fats, waxes, sterols, fat-soluble vitamins (such as vitamins A, D, E and K), monoglycerides, diglycerides, phospholipids, and others. The functions of lipids include storing ...
s and carotenoid
Carotenoids () are yellow, orange, and red organic pigments that are produced by plants and algae, as well as several bacteria, archaea, and fungi. Carotenoids give the characteristic color to pumpkins, carrots, parsnips, corn, tomatoes, cana ...
s and the processes of photosynthesis
Photosynthesis ( ) is a system of biological processes by which photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical energy necessary to fuel their metabo ...
and oxidative phosphorylation
Oxidative phosphorylation(UK , US : or electron transport-linked phosphorylation or terminal oxidation, is the metabolic pathway in which Cell (biology), cells use enzymes to Redox, oxidize nutrients, thereby releasing chemical energy in order ...
.
Discovery and design
 New drugs are the products of a long
New drugs are the products of a long drug development
Drug development is the process of bringing a new pharmaceutical drug to the market once a lead compound has been identified through the process of drug discovery. It includes preclinical research on microorganisms and animals, filing for regu ...
process, the first step of which is often the discovery of a new enzyme inhibitor. There are two principle approaches of discovering these inhibitors.
The first general method is rational drug design
Drug design, often referred to as rational drug design or simply rational design, is the invention, inventive process of finding new medications based on the knowledge of a biological target. The drug is most commonly an organic compound, organi ...
based on mimicking the transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked w ...
of the chemical reaction catalysed by the enzyme. The designed inhibitor often closely resembles the substrate, except that the portion of the substrate that undergoes chemical reaction is replaced by a chemically stable functional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
that resembles the transition state. Since the enzyme has evolved to stabilise the transition state, transition state analogues generally possess higher affinity for the enzyme compared to the substrate, and therefore are effective inhibitors.
The second way of discovering new enzyme inhibitors is high-throughput screening
High-throughput screening (HTS) is a method for scientific discovery especially used in drug discovery and relevant to the fields of biology, materials science and chemistry. Using robotics, data processing/control software, liquid handling device ...
of large libraries of structurally diverse compounds to identify hit molecules that bind to the enzyme. This method has been extended to include virtual screening
Virtual screening (VS) is a computational technique used in drug discovery to search libraries of small molecules in order to identify those structures which are most likely to bind to a drug target, typically a protein receptor (biochemistry), r ...
of databases of diverse molecules using computers, which are then followed by experimental confirmation of binding of the virtual screening hits. Complementary approaches that can provide new starting points for inhibitors include fragment-based lead discovery
Fragment-based lead discovery (FBLD) also known as fragment-based drug discovery (FBDD) is a method used for finding lead compounds as part of the drug discovery process. Fragments are small organic molecules which are small in size and low in mol ...
and DNA Encoded Chemical Libraries (DEL).
Hits from any of the above approaches can be optimised to high affinity binders that efficiently inhibit the enzyme. Computer-based methods for predicting the binding orientation and affinity of an inhibitor for an enzyme such as molecular docking and molecular mechanics
Molecular mechanics uses classical mechanics to model molecular systems. The Born–Oppenheimer approximation is assumed valid and the potential energy of all systems is calculated as a function of the nuclear coordinates using Force field (chemi ...
can be used to assist in the optimisation process. New inhibitors are used to obtain crystallographic structures of the enzyme in an inhibitor/enzyme complex to show how the molecule is binding to the active site, allowing changes to be made to the inhibitor to optimise binding in a process known as structure-based drug design. This test and improve cycle is repeated until a sufficiently potent inhibitor is produced.
See also
* Activity-based proteomics – a branch ofproteomics
Proteomics is the large-scale study of proteins. Proteins are vital macromolecules of all living organisms, with many functions such as the formation of structural fibers of muscle tissue, enzymatic digestion of food, or synthesis and replicatio ...
that uses covalent enzyme inhibitors as reporters to monitor enzyme activity.
*Antimetabolite
An antimetabolite is a chemical that inhibits the use of a metabolite, which is another chemical that is part of normal metabolism. Such substances are often similar in structure to the metabolite that they interfere with, such as the antifolat ...
– an enzyme inhibitor that is used to interfere with cell growth and division
* Transition state analogue – a type of enzyme inhibitor that mimics the transition state of the chemical reaction catalysed by the enzyme
References
External links
*, Database of enzymes giving lists of known inhibitors for each entry * Database of drugs and enzyme inhibitors * Recommendations of the Nomenclature Committee of the International Union of Biochemistry (NC-IUB) on enzyme inhibition terminology {{Authority control Biochemical reactions Medicinal chemistry Metabolism