Prion on:
[Wikipedia]
[Google]
[Amazon]
A prion () is a misfolded protein that induces misfolding in normal variants of the same protein, leading to cellular death. Prions are responsible for prion diseases, known as transmissible spongiform encephalopathy (TSEs), which are fatal and transmissible neurodegenerative diseases affecting both humans and animals. These proteins can misfold sporadically, due to genetic mutations, or by exposure to an already misfolded protein, leading to an abnormal three-dimensional structure that can propagate misfolding in other proteins.
The term ''prion'' comes from "proteinaceous infectious particle". Unlike other infectious agents such as viruses, bacteria, and fungi, prions do not contain
Lay summary: in reference to its ability to self-propagate and transmit its conformation to other proteins. Its main pronunciation is , although , as the homographic name of the bird (prions or whalebirds) is pronounced, is also heard. In his 1982 paper introducing the term, Prusiner specified that it is "pronounced ''pree-on''".
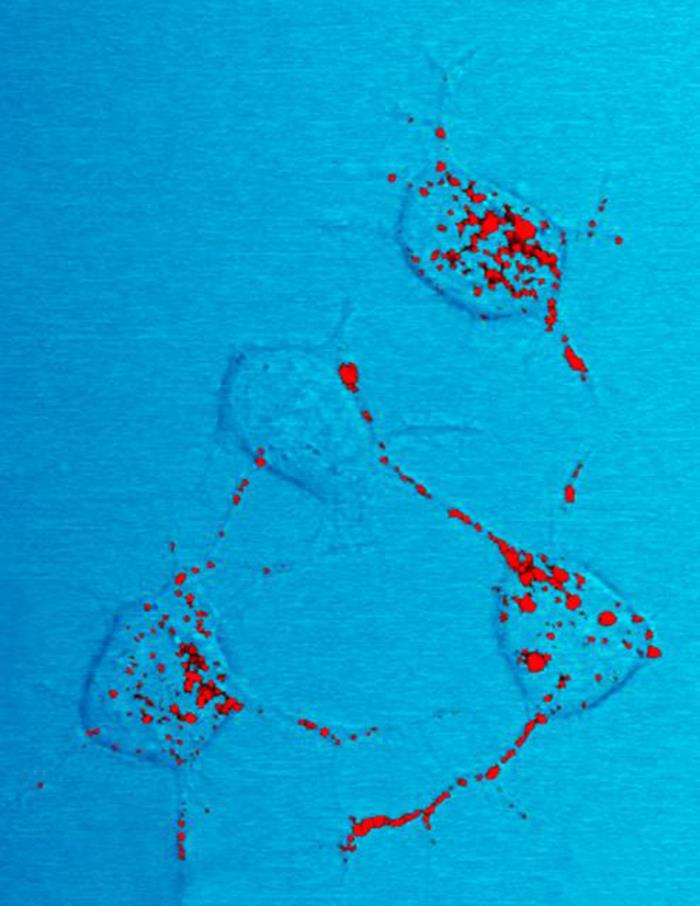 The infectious isoform of PrP, known as PrPSc, or simply the prion, is able to convert normal PrPC proteins into the infectious isoform by changing their conformation, or shape; this, in turn, alters the way the proteins
The infectious isoform of PrP, known as PrPSc, or simply the prion, is able to convert normal PrPC proteins into the infectious isoform by changing their conformation, or shape; this, in turn, alters the way the proteins


 The first hypothesis that tried to explain how prions replicate in a protein-only manner was the heterodimer model. This model assumed that a single PrPSc molecule binds to a single PrPC molecule and catalyzes its conversion into PrPSc. The two PrPSc molecules then come apart and can go on to convert more PrPC. However, a model of prion replication must explain both how prions propagate, and why their spontaneous appearance is so rare. Manfred Eigen showed that the heterodimer model requires PrPSc to be an extraordinarily effective catalyst, increasing the rate of the conversion reaction by a factor of around 1015. This problem does not arise if PrPSc exists only in aggregated forms such as amyloid, where cooperativity may act as a barrier to spontaneous conversion. What is more, despite considerable effort, infectious monomeric PrPSc has never been isolated.
An alternative model assumes that PrPSc exists only as fibrils, and that fibril ends bind PrPC and convert it into PrPSc. If this were all, then the quantity of prions would increase linearly, forming ever longer fibrils. But
The first hypothesis that tried to explain how prions replicate in a protein-only manner was the heterodimer model. This model assumed that a single PrPSc molecule binds to a single PrPC molecule and catalyzes its conversion into PrPSc. The two PrPSc molecules then come apart and can go on to convert more PrPC. However, a model of prion replication must explain both how prions propagate, and why their spontaneous appearance is so rare. Manfred Eigen showed that the heterodimer model requires PrPSc to be an extraordinarily effective catalyst, increasing the rate of the conversion reaction by a factor of around 1015. This problem does not arise if PrPSc exists only in aggregated forms such as amyloid, where cooperativity may act as a barrier to spontaneous conversion. What is more, despite considerable effort, infectious monomeric PrPSc has never been isolated.
An alternative model assumes that PrPSc exists only as fibrils, and that fibril ends bind PrPC and convert it into PrPSc. If this were all, then the quantity of prions would increase linearly, forming ever longer fibrils. But
Lay summary: this report was retracted in 2024. Preliminary evidence supporting the notion that prions can be transmitted through use of urine-derived human menopausal gonadotropin, administered for the treatment of
World Health Organisation
– WHO information on prion diseases
The UK BSE Inquiry
nbsp;– Report of the UK public inquiry into BSE and variant CJD
UK Spongiform Encephalopathy Advisory Committee (SEAC)
* * {{Authority control Infectious diseases Amyloidosis
nucleic acid
Nucleic acids are large biomolecules that are crucial in all cells and viruses. They are composed of nucleotides, which are the monomer components: a pentose, 5-carbon sugar, a phosphate group and a nitrogenous base. The two main classes of nuclei ...
s ( DNA or RNA
Ribonucleic acid (RNA) is a polymeric molecule that is essential for most biological functions, either by performing the function itself (non-coding RNA) or by forming a template for the production of proteins (messenger RNA). RNA and deoxyrib ...
). Prions are mainly twisted isoforms of the major prion protein (PrP), a naturally occurring protein with an uncertain function. They are the hypothesized cause of various TSEs, including scrapie in sheep, chronic wasting disease (CWD) in deer, bovine spongiform encephalopathy (BSE) in cattle (mad cow disease), and Creutzfeldt–Jakob disease (CJD) in humans.
All known prion diseases in mammal
A mammal () is a vertebrate animal of the Class (biology), class Mammalia (). Mammals are characterised by the presence of milk-producing mammary glands for feeding their young, a broad neocortex region of the brain, fur or hair, and three ...
s affect the structure of the brain
The brain is an organ (biology), organ that serves as the center of the nervous system in all vertebrate and most invertebrate animals. It consists of nervous tissue and is typically located in the head (cephalization), usually near organs for ...
or other neural tissues. These diseases are progressive, have no known effective treatment, and are invariably fatal. Most prion diseases were thought to be caused by PrP until 2015 when a prion form of alpha-synuclein was linked to multiple system atrophy (MSA). Misfolded proteins are also linked to other neurodegenerative diseases like Alzheimer's disease
Alzheimer's disease (AD) is a neurodegenerative disease and the cause of 60–70% of cases of dementia. The most common early symptom is difficulty in remembering recent events. As the disease advances, symptoms can include problems wit ...
, Parkinson's disease
Parkinson's disease (PD), or simply Parkinson's, is a neurodegenerative disease primarily of the central nervous system, affecting both motor system, motor and non-motor systems. Symptoms typically develop gradually and non-motor issues become ...
, and amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS), also known as motor neuron disease (MND) or—in the United States—Lou Gehrig's disease (LGD), is a rare, Terminal illness, terminal neurodegenerative disease, neurodegenerative disorder that results i ...
(ALS), which are sometimes referred to as ''prion-like diseases''.
Prions are a type of intrinsically disordered protein that continuously changes conformation unless bound to a specific partner, such as another protein. Once a prion binds to another in the same conformation, it stabilizes and can form a fibril, leading to abnormal protein aggregates called amyloids. These amyloids accumulate in infected tissue, causing damage and cell death. The structural stability of prions makes them resistant to denaturation by chemical or physical agents, complicating disposal and containment, and raising concerns about iatrogenic spread through medical instruments.
Etymology and pronunciation
The word ''prion'', coined in 1982 by Stanley B. Prusiner, is derived from protein and infection, hence prion, and is short for "proteinaceous infectious particle",Lay summary: in reference to its ability to self-propagate and transmit its conformation to other proteins. Its main pronunciation is , although , as the homographic name of the bird (prions or whalebirds) is pronounced, is also heard. In his 1982 paper introducing the term, Prusiner specified that it is "pronounced ''pree-on''".
Prion protein
Structure
Prions consist of a misfolded form of major prion protein (PrP), a protein that is a natural part of the bodies of humans and other animals. The PrP found in infectious prions has a differentstructure
A structure is an arrangement and organization of interrelated elements in a material object or system, or the object or system so organized. Material structures include man-made objects such as buildings and machines and natural objects such as ...
and is resistant to proteases, the enzymes in the body that can normally break down proteins. The normal form of the protein is called PrPC, while the infectious form is called PrPSc – the ''C'' refers to 'cellular' PrP, while the ''Sc'' refers to ' scrapie', the prototypic prion disease, occurring in sheep. PrP can also be induced to fold into other more-or-less well-defined isoforms in vitro; although their relationships to the form(s) that are pathogenic in vivo is often unclear, high-resolution structural analyses have begun to reveal structural features that correlate with prion infectivity.
PrPC
PrPC is a normal protein found on the membranes of cells, "including several blood components of which platelets constitute the largest reservoir in humans." It has 209amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 a ...
s (in humans), one disulfide bond, a molecular mass of 35–36 kDa and a mainly alpha-helical structure. Several topological forms exist; one cell surface form anchored via glycolipid and two transmembrane
A transmembrane protein is a type of integral membrane protein that spans the entirety of the cell membrane. Many transmembrane proteins function as gateways to permit the transport of specific substances across the membrane. They frequently u ...
forms. The normal protein is not sedimentable; meaning that it cannot be separated by centrifuging techniques. It has a complex function, which continues to be investigated. PrPC binds copper
Copper is a chemical element; it has symbol Cu (from Latin ) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orang ...
(II) ions (those in a +2 oxidation state) with high affinity. This property is supposed to play a role in PrPC’s anti-oxidative properties via reversible oxidation
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
of the N-terminal’s methionine residues into sulfoxide. Moreover, studies have suggested that, in vivo, due to PrPC’s low selectivity to metallic substrates, the protein’s anti oxidative function is impaired when in contact with metals other than copper
Copper is a chemical element; it has symbol Cu (from Latin ) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orang ...
. PrPC is readily digested by proteinase K and can be liberated from the cell surface by the enzyme phosphoinositide phospholipase C (PI-PLC), which cleaves the glycophosphatidylinositol (GPI) glycolipid anchor. PrP plays an important role in cell-cell adhesion and intracellular signaling ''in vivo'', and may therefore be involved in cell-cell communication in the brain.
PrPSc
 The infectious isoform of PrP, known as PrPSc, or simply the prion, is able to convert normal PrPC proteins into the infectious isoform by changing their conformation, or shape; this, in turn, alters the way the proteins
The infectious isoform of PrP, known as PrPSc, or simply the prion, is able to convert normal PrPC proteins into the infectious isoform by changing their conformation, or shape; this, in turn, alters the way the proteins interconnect
In telecommunications, interconnection is the physical linking of a carrier's network with equipment or facilities not belonging to that network. The term may refer to a connection between a carrier's facilities and the equipment belonging to its ...
. PrPSc always causes prion disease. PrPSc has a higher proportion of β-sheet
The beta sheet (β-sheet, also β-pleated sheet) is a common structural motif, motif of the regular protein secondary structure. Beta sheets consist of beta strands (β-strands) connected laterally by at least two or three backbone chain, backbon ...
structure in place of the normal α-helix structure. Several highly infectious, brain-derived PrPSc structures have been discovered by cryo-electron microscopy. Another brain-derived fibril structure isolated from humans with Gerstmann-Straussler-Schienker syndrome has also been determined. All of the structures described in high resolution so far are amyloid fibers in which individual PrP molecules are stacked via intermolecular beta sheets. However, 2-D crystalline arrays have also been reported at lower resolution in ''ex vivo'' preparations of prions. In the prion amyloids, the glycolipid anchors and asparagine-linked glycans, when present, project outward from the lateral surfaces of the fiber cores. Often PrPSc is bound to cellular membranes, presumably via its array of glycolipid anchors, however, sometimes the fibers are dissociated from membranes and accumulate outside of cells in the form of plaques. The end of each fiber acts as a template onto which free protein molecules may attach, allowing the fiber to grow. This growth process requires complete refolding of PrPC. Different prion strains have distinct templates, or conformations, even when composed of PrP molecules of the same amino acid sequence, as occurs in a particular host genotype
The genotype of an organism is its complete set of genetic material. Genotype can also be used to refer to the alleles or variants an individual carries in a particular gene or genetic location. The number of alleles an individual can have in a ...
. Under most circumstances, only PrP molecules with an identical amino acid sequence to the infectious PrPSc are incorporated into the growing fiber. However, cross-species transmission also happens rarely.
PrPres
Protease-resistant PrPSc-like protein (PrPres) is the name given to any isoform of PrPc which is structurally altered and converted into a misfolded proteinase K-resistant form. To model conversion of PrPC to PrPSc ''in vitro'', Kocisko ''et al''. showed that PrPSc could cause PrPC to convert to PrPres under cell-free conditions and Soto ''et al''. demonstrated sustained amplification of PrPres and prion infectivity by a procedure involving cyclic amplification of protein misfolding. The term "PrPres" may refer either to protease-resistant forms of PrPSc, which is isolated from infectious tissue and associated with the transmissible spongiform encephalopathy agent, or to other protease-resistant forms of PrP that, for example, might be generated ''in vitro''. Accordingly, unlike PrPSc, PrPres may not necessarily be infectious.
Normal function of PrP
The physiological function of the prion protein remains poorly understood. While data from in vitro experiments suggest many dissimilar roles, studies on PrP knockout mice have provided only limited information because these animals exhibit only minor abnormalities. In research done in mice, it was found that the cleavage of PrP in peripheral nerves causes the activation of myelin repair inSchwann cells
Schwann cells or neurolemmocytes (named after German physiologist Theodor Schwann) are the principal glia of the peripheral nervous system (PNS). Glial cells function to support neurons and in the PNS, also include Satellite glial cell, satellite ...
and that the lack of PrP proteins caused demyelination in those cells.
PrP and regulated cell death
MAVS, RIP1, and RIP3 are prion-like proteins found in other parts of the body. They also polymerise into filamentous amyloid fibers which initiate regulated cell death in the case of a viral infection to prevent the spread of virions to other, surrounding cells.PrP and long-term memory
A review of evidence in 2005 suggested that PrP may have a normal function in the maintenance of long-term memory. As well, a 2004 study found that mice lacking genes for normal cellular PrP protein show altered hippocampallong-term potentiation
In neuroscience, long-term potentiation (LTP) is a persistent strengthening of synapses based on recent patterns of activity. These are patterns of synaptic activity that produce a long-lasting increase in signal transmission between two neuron ...
. A recent study that also suggests why this might be the case, found that neuronal protein CPEB has a similar genetic sequence to yeast prion proteins. The prion-like formation of CPEB is essential for maintaining long-term synaptic changes associated with long-term memory formation.
PrP and stem cell renewal
A 2006 article from the Whitehead Institute for Biomedical Research indicates that PrP expression on stem cells is necessary for an organism's self-renewal of bone marrow. The study showed that all long-term hematopoietic stem cells express PrP on their cell membrane and that hematopoietic tissues with PrP-null stem cells exhibit increased sensitivity to cell depletion.PrP and innate immunity
There is some evidence that PrP may play a role in innate immunity, as the expression of PRNP, the PrP gene, is upregulated in many viral infections and PrP has antiviral properties against many viruses, including HIV.Replication
 The first hypothesis that tried to explain how prions replicate in a protein-only manner was the heterodimer model. This model assumed that a single PrPSc molecule binds to a single PrPC molecule and catalyzes its conversion into PrPSc. The two PrPSc molecules then come apart and can go on to convert more PrPC. However, a model of prion replication must explain both how prions propagate, and why their spontaneous appearance is so rare. Manfred Eigen showed that the heterodimer model requires PrPSc to be an extraordinarily effective catalyst, increasing the rate of the conversion reaction by a factor of around 1015. This problem does not arise if PrPSc exists only in aggregated forms such as amyloid, where cooperativity may act as a barrier to spontaneous conversion. What is more, despite considerable effort, infectious monomeric PrPSc has never been isolated.
An alternative model assumes that PrPSc exists only as fibrils, and that fibril ends bind PrPC and convert it into PrPSc. If this were all, then the quantity of prions would increase linearly, forming ever longer fibrils. But
The first hypothesis that tried to explain how prions replicate in a protein-only manner was the heterodimer model. This model assumed that a single PrPSc molecule binds to a single PrPC molecule and catalyzes its conversion into PrPSc. The two PrPSc molecules then come apart and can go on to convert more PrPC. However, a model of prion replication must explain both how prions propagate, and why their spontaneous appearance is so rare. Manfred Eigen showed that the heterodimer model requires PrPSc to be an extraordinarily effective catalyst, increasing the rate of the conversion reaction by a factor of around 1015. This problem does not arise if PrPSc exists only in aggregated forms such as amyloid, where cooperativity may act as a barrier to spontaneous conversion. What is more, despite considerable effort, infectious monomeric PrPSc has never been isolated.
An alternative model assumes that PrPSc exists only as fibrils, and that fibril ends bind PrPC and convert it into PrPSc. If this were all, then the quantity of prions would increase linearly, forming ever longer fibrils. But exponential growth
Exponential growth occurs when a quantity grows as an exponential function of time. The quantity grows at a rate directly proportional to its present size. For example, when it is 3 times as big as it is now, it will be growing 3 times as fast ...
of both PrPSc and the quantity of infectious particles is observed during prion disease. This can be explained by taking into account fibril breakage. A mathematical solution for the exponential growth rate resulting from the combination of fibril growth and fibril breakage has been found. The exponential growth rate depends largely on the square root
In mathematics, a square root of a number is a number such that y^2 = x; in other words, a number whose ''square'' (the result of multiplying the number by itself, or y \cdot y) is . For example, 4 and −4 are square roots of 16 because 4 ...
of the PrPC concentration. The incubation period is determined by the exponential growth rate, and in vivo data on prion diseases in transgenic mice match this prediction. The same square root dependence is also seen in vitro
''In vitro'' (meaning ''in glass'', or ''in the glass'') Research, studies are performed with Cell (biology), cells or biological molecules outside their normal biological context. Colloquially called "test-tube experiments", these studies in ...
in experiments with a variety of different amyloid proteins.
The mechanism of prion replication has implications for designing drugs. Since the incubation period of prion diseases is so long, an effective drug does not need to eliminate all prions, but simply needs to slow down the rate of exponential growth. Models predict that the most effective way to achieve this, using a drug with the lowest possible dose, is to find a drug that binds to fibril ends and blocks them from growing any further.
Researchers at Dartmouth College discovered that endogenous host cofactor molecules such as the phospholipid molecule (e.g. phosphatidylethanolamine) and polyanions (e.g. single stranded RNA molecules) are necessary to form PrPSc molecules with high levels of specific infectivity ''in vitro'', whereas protein-only PrPSc molecules appear to lack significant levels of biological infectivity.
Transmissible spongiform encephalopathies
Prions cause neurodegenerative disease by aggregating extracellularly within thecentral nervous system
The central nervous system (CNS) is the part of the nervous system consisting primarily of the brain, spinal cord and retina. The CNS is so named because the brain integrates the received information and coordinates and influences the activity o ...
to form plaques known as amyloids, which disrupt the normal tissue structure. This disruption is characterized by "holes" in the tissue with resultant spongy architecture due to the vacuole
A vacuole () is a membrane-bound organelle which is present in Plant cell, plant and Fungus, fungal Cell (biology), cells and some protist, animal, and bacterial cells. Vacuoles are essentially enclosed compartments which are filled with water ...
formation in the neurons. Other histological changes include astrogliosis and the absence of an inflammatory reaction. While the incubation period for prion diseases is relatively long (5 to 20 years), once symptoms appear the disease progresses rapidly, leading to brain damage and death. Neurodegenerative symptoms can include convulsions, dementia
Dementia is a syndrome associated with many neurodegenerative diseases, characterized by a general decline in cognitive abilities that affects a person's ability to perform activities of daily living, everyday activities. This typically invo ...
, ataxia
Ataxia (from Greek α- negative prefix+ -τάξις rder= "lack of order") is a neurological sign consisting of lack of voluntary coordination of muscle movements that can include gait abnormality, speech changes, and abnormalities in e ...
(balance and coordination dysfunction), and behavioural or personality changes.
Many different mammalian species can be affected by prion diseases, as the prion protein (PrP) is very similar in all mammals. Due to small differences in PrP between different species it is unusual for a prion disease to transmit from one species to another. The human prion disease variant Creutzfeldt–Jakob disease, however, is thought to be caused by a prion that typically infects cattle, causing bovine spongiform encephalopathy and is transmitted through infected meat.
All known prion diseases are untreatable and fatal.
Until 2015 all known mammalian prion diseases were considered to be caused by the prion protein, PrP. After 2015 this remains true for diseases in the category of "transmissible spongiform encephalopathy" (TSE), which is transmissible and causes a specific sponge-like appearance of infected brain tissue. The endogenous, properly folded form of the prion protein is denoted PrPC (for ''Common'' or ''Cellular''), whereas the disease-linked, misfolded form is denoted PrPSc (for ''Scrapie''), after one of the diseases first linked to prions and neurodegeneration. The precise structure of the prion is not known, though they can be formed spontaneously by combining PrPC, homopolymeric polyadenylic acid, and lipids in a protein misfolding cyclic amplification (PMCA) reaction even in the absence of pre-existing infectious prions. This result is further evidence that prion replication does not require genetic information.
Transmission
It has been recognized that prion diseases can arise in three different ways: acquired, familial, or sporadic. It is often assumed that the diseased form directly interacts with the normal form to make it rearrange its structure. One idea, the "Protein X" hypothesis, is that an as-yet unidentified cellular protein (Protein X) enables the conversion of PrPC to PrPSc by bringing a molecule of each of the two together into a complex. The primary method of infection in animals is through ingestion. It is thought that prions may be deposited in the environment through the remains of dead animals and via urine, saliva, and other body fluids. They may then linger in the soil by binding to clay and other minerals. AUniversity of California
The University of California (UC) is a public university, public Land-grant university, land-grant research university, research university system in the U.S. state of California. Headquartered in Oakland, California, Oakland, the system is co ...
research team has provided evidence for the theory that infection can occur from prions in manure. And, since manure is present in many areas surrounding water reservoirs, as well as used on many crop fields, it raises the possibility of widespread transmission. Although it was initially reported in January 2011 that researchers had discovered prions spreading through airborne transmission on aerosol
An aerosol is a suspension (chemistry), suspension of fine solid particles or liquid Drop (liquid), droplets in air or another gas. Aerosols can be generated from natural or Human impact on the environment, human causes. The term ''aerosol'' co ...
particles in an animal testing
Animal testing, also known as animal experimentation, animal research, and ''in vivo'' testing, is the use of animals, as model organisms, in experiments that seek answers to scientific and medical questions. This approach can be contrasted ...
experiment focusing on scrapie infection in laboratory mice,Lay summary: this report was retracted in 2024. Preliminary evidence supporting the notion that prions can be transmitted through use of urine-derived human menopausal gonadotropin, administered for the treatment of
infertility
In biology, infertility is the inability of a male and female organism to Sexual reproduction, reproduce. It is usually not the natural state of a healthy organism that has reached sexual maturity, so children who have not undergone puberty, whi ...
, was published in 2011.
Genetic susceptibility
The majority of human prion diseases are classified as sporadic Creutzfeldt–Jakob disease (sCJD). Genetic research has identified an association between susceptibility to sCJD and a polymorphism at codon 129 in the PRNP gene, which encodes the prion protein (PrP). A homozygous methionine/methionine (MM) genotype at this position has been shown to significantly increase the risk of developing sCJD when compared to a heterozygous methionine/valine (MV) genotype. Analysis of multiple studies has shown that individuals with the MM genotype are approximately five times more likely to develop sCJD than those with the MV genotype.Prions in plants
In 2015, researchers at The University of Texas Health Science Center at Houston found that plants can be a vector for prions. When researchers fed hamsters grass that grew on ground where a deer that died with chronic wasting disease (CWD) was buried, the hamsters became ill with CWD, suggesting that prions can bind to plants, which then take them up into the leaf and stem structure, where they can be eaten by herbivores, thus completing the cycle. It is thus possible that there is a progressively accumulating number of prions in the environment.Sterilization
Infectious particles possessingnucleic acid
Nucleic acids are large biomolecules that are crucial in all cells and viruses. They are composed of nucleotides, which are the monomer components: a pentose, 5-carbon sugar, a phosphate group and a nitrogenous base. The two main classes of nuclei ...
are dependent upon it to direct their continued replication. Prions, however, are infectious by their effect on normal versions of the protein. Sterilizing prions, therefore, requires the denaturation of the protein to a state in which the molecule is no longer able to induce the abnormal folding of normal proteins. In general, prions are quite resistant to proteases, heat, ionizing radiation
Ionizing (ionising) radiation, including Radioactive decay, nuclear radiation, consists of subatomic particles or electromagnetic waves that have enough energy per individual photon or particle to ionization, ionize atoms or molecules by detaching ...
, and formaldehyde treatments, although their infectivity can be reduced by such treatments. Effective prion decontamination relies upon protein hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
or reduction or destruction of protein tertiary structure. Examples include sodium hypochlorite, sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula . It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly corrosive base (chemistry), ...
, and strongly acidic detergents such as LpH.
The World Health Organization
The World Health Organization (WHO) is a list of specialized agencies of the United Nations, specialized agency of the United Nations which coordinates responses to international public health issues and emergencies. It is headquartered in Gen ...
recommends any of the following three procedures for the sterilization of all heat-resistant surgical instruments to ensure that they are not contaminated with prions:
# Immerse in 1N sodium hydroxide and place in a gravity-displacement autoclave at 121 °C for 30 minutes; clean; rinse in water; and then perform routine sterilization processes.
# Immerse in 1N sodium hypochlorite (20,000 parts per million available chlorine) for 1 hour; transfer instruments to water; heat in a gravity-displacement autoclave at 121 °C for 1 hour; clean; and then perform routine sterilization processes.
# Immerse in 1N sodium hydroxide or sodium hypochlorite (20,000 parts per million available chlorine) for 1 hour; remove and rinse in water, then transfer to an open pan and heat in a gravity-displacement (121 °C) or in a porous-load (134 °C) autoclave for 1 hour; clean; and then perform routine sterilization processes.
for 18 minutes in a pressurized steam autoclave has been found to be somewhat effective in deactivating the agent of disease. Ozone
Ozone () (or trioxygen) is an Inorganic compound, inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , break ...
sterilization has been studied as a potential method for prion denaturation and deactivation. Other approaches being developed include thiourea-urea
Urea, also called carbamide (because it is a diamide of carbonic acid), is an organic compound with chemical formula . This amide has two Amine, amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest am ...
treatment, guanidinium chloride treatment, and special heat-resistant subtilisin combined with heat and detergent. A number of decontamination reagents have been commercially manufactured with significant differences in efficacy among methods. A method sufficient for sterilizing prions on one material may fail on another.
Renaturation of a completely denatured prion to infectious status has not yet been achieved; however, partially denatured prions can be renatured to an infective status under certain artificial conditions.
Degradation resistance in nature
Overwhelming evidence shows that prions resist degradation and persist in the environment for years, and proteases do not degrade them. Experimental evidence shows that ''unbound'' prions degrade over time, while soil-bound prions remain at stable or increasing levels, suggesting that prions likely accumulate in the environment. One 2015 study by US scientists found that repeated drying and wetting may render soil bound prions less infectious, although this was dependent on the soil type they were bound to.Degradation by living beings
More recent studies suggest scrapie prions can be degraded by diverse cellular machinery of the affected animal cell. In an infected cell, extracellular lysosomal PrPSc does not tend to accumulate and is rapidly cleared by thelysosome
A lysosome () is a membrane-bound organelle that is found in all mammalian cells, with the exception of red blood cells (erythrocytes). There are normally hundreds of lysosomes in the cytosol, where they function as the cell’s degradation cent ...
via the endosome. The intracellular portion is harder to clear and tends to build up. The ubiquitin proteasome system appears to be able to degrade small enough aggregates. Autophagy plays a bigger role by accepting PrPSc from the ER lumen and degrading it. Altogether these mechanisms allow the cell to delay its death from being overwhelmed by misfolded proteins. Inhibition of autophagy accelerates prion accumulation whereas encouragement of autophagy promotes prion clearance. Some autophagy-promoting compounds have shown promise in animal models by delaying disease onset and death.
In addition, keratinase from ''B. licheniformis'', alkaline serine protease from ''Streptomyces sp'', subtilisin-like pernisine from '' Aeropyrum pernix'', alkaline protease from '' Nocardiopsis sp'', nattokinase from '' B. subtilis'', engineered subtilisins from ''B. lentus'' and serine protease from three lichen species have been found to degrade PrPSc.
Fungi
Proteins showing prion-type behavior are also found in somefungi
A fungus (: fungi , , , or ; or funguses) is any member of the group of eukaryotic organisms that includes microorganisms such as yeasts and mold (fungus), molds, as well as the more familiar mushrooms. These organisms are classified as one ...
, which has been useful in helping to understand mammalian prions. Fungal prions do not always cause disease in their hosts. In yeast, protein refolding to the prion configuration is assisted by chaperone proteins such as Hsp104. All known prions induce the formation of an amyloid fold, in which the protein polymerises into an aggregate consisting of tightly packed beta sheets. Amyloid aggregates are fibrils, growing at their ends, and replicate when breakage causes two growing ends to become four growing ends. The incubation period of prion diseases is determined by the exponential growth
Exponential growth occurs when a quantity grows as an exponential function of time. The quantity grows at a rate directly proportional to its present size. For example, when it is 3 times as big as it is now, it will be growing 3 times as fast ...
rate associated with prion replication, which is a balance between the linear growth and the breakage of aggregates.
Fungal proteins that exhibit templated structural change were discovered in the yeast ''Saccharomyces cerevisiae
''Saccharomyces cerevisiae'' () (brewer's yeast or baker's yeast) is a species of yeast (single-celled fungal microorganisms). The species has been instrumental in winemaking, baking, and brewing since ancient times. It is believed to have be ...
'' by Reed Wickner in the early 1990s. For their mechanistic similarity to mammalian prions, they were termed yeast prions. Subsequent to this, a prion has also been found in the fungus '' Podospora anserina''. These prions behave similarly to PrP, but, in general, are nontoxic to their hosts. Susan Lindquist's group at the Whitehead Institute has argued some of the fungal prions are not associated with any disease state, but may have a useful role; however, researchers at the NIH have also provided arguments suggesting that fungal prions could be considered a diseased state. There is evidence that fungal proteins have evolved specific functions that are beneficial to the microorganism that enhance their ability to adapt to their diverse environments. Further, within yeasts, prions can act as vectors of epigenetic inheritance, transferring traits to offspring without any genomic change.
Research into fungal prions has given strong support to the protein-only concept, since purified protein extracted from cells with a prion state has been demonstrated to convert the normal form of the protein into a misfolded form ''in vitro
''In vitro'' (meaning ''in glass'', or ''in the glass'') Research, studies are performed with Cell (biology), cells or biological molecules outside their normal biological context. Colloquially called "test-tube experiments", these studies in ...
'', and in the process, preserve the information corresponding to different strains of the prion state. It has also shed some light on prion domains, which are regions in a protein that promote the conversion into a prion. Fungal prions have helped to suggest mechanisms of conversion that may apply to all prions, though fungal prions appear distinct from infectious mammalian prions in the lack of cofactor required for propagation. The characteristic prion domains may vary between species – e.g., characteristic fungal prion domains are not found in mammalian prions.
Treatments
There are no effective treatments for prion diseases as of 2018. Clinical trials in humans have not met with success and have been hampered by the rarity of prion diseases. Many possible treatments work in the test-tube but not in lab animals. One treatment that prolongs the incubation period in lab mice has failed in human patients diagnosed with definite or probable vCJD. Another treatment that works in mice was only tried in 6 human patients before it went out of stock, all of which died. There was no significant increase in lifespan, but autopsy suggests that the drug has partially worked. While there is no known way to extend the life of a prion disease patient, some drugs can be prescribed to control specific symptoms of the disease and accommodations can be given to improve quality of life.In other diseases
Prion-like domains have been found in a variety of other mammalian proteins. Some of these proteins have been implicated in the ontogeny of age-related neurodegenerative disorders such asamyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS), also known as motor neuron disease (MND) or—in the United States—Lou Gehrig's disease (LGD), is a rare, Terminal illness, terminal neurodegenerative disease, neurodegenerative disorder that results i ...
(ALS), frontotemporal lobar degeneration with ubiquitin-positive inclusions (FTLD-U), Alzheimer's disease
Alzheimer's disease (AD) is a neurodegenerative disease and the cause of 60–70% of cases of dementia. The most common early symptom is difficulty in remembering recent events. As the disease advances, symptoms can include problems wit ...
, Parkinson's disease
Parkinson's disease (PD), or simply Parkinson's, is a neurodegenerative disease primarily of the central nervous system, affecting both motor system, motor and non-motor systems. Symptoms typically develop gradually and non-motor issues become ...
, and Huntington's disease. They are also implicated in some forms of systemic amyloidosis including AA amyloidosis that develops in humans and animals with inflammatory and infectious diseases such as tuberculosis
Tuberculosis (TB), also known colloquially as the "white death", or historically as consumption, is a contagious disease usually caused by ''Mycobacterium tuberculosis'' (MTB) bacteria. Tuberculosis generally affects the lungs, but it can al ...
, Crohn's disease
Crohn's disease is a type of inflammatory bowel disease (IBD) that may affect any segment of the gastrointestinal tract. Symptoms often include abdominal pain, diarrhea, fever, abdominal distension, and weight loss. Complications outside of the ...
, rheumatoid arthritis
Rheumatoid arthritis (RA) is a long-term autoimmune disorder that primarily affects synovial joint, joints. It typically results in warm, swollen, and painful joints. Pain and stiffness often worsen following rest. Most commonly, the wrist and h ...
, and HIV/AIDS
The HIV, human immunodeficiency virus (HIV) is a retrovirus that attacks the immune system. Without treatment, it can lead to a spectrum of conditions including acquired immunodeficiency syndrome (AIDS). It is a Preventive healthcare, pr ...
. AA amyloidosis, like prion disease, may be transmissible. This has given rise to the 'prion paradigm', where otherwise harmless proteins can be converted to a pathogenic form by a small number of misfolded, nucleating proteins.
The definition of a prion-like domain arises from the study of fungal prions. In yeast, prionogenic proteins have a portable prion domain that is both necessary and sufficient for self-templating and protein aggregation. This has been shown by attaching the prion domain to a reporter protein, which then aggregates like a known prion. Similarly, removing the prion domain from a fungal prion protein inhibits prionogenesis. This modular view of prion behaviour has led to the hypothesis that similar prion domains are present in animal proteins, in addition to PrP. These fungal prion domains have several characteristic sequence features. They are typically enriched in asparagine, glutamine, tyrosine and glycine residues, with an asparagine bias being particularly conducive to the aggregative property of prions. Historically, prionogenesis has been seen as independent of sequence and only dependent on relative residue content. However, this has been shown to be false, with the spacing of prolines and charged residues having been shown to be critical in amyloid formation.
Bioinformatic screens have predicted that over 250 human proteins contain prion-like domains (PrLD). These domains are hypothesized to have the same transmissible, amyloidogenic properties of PrP and known fungal proteins. As in yeast, proteins involved in gene expression and RNA binding seem to be particularly enriched in PrLD's, compared to other classes of protein. In particular, 29 of the known 210 proteins with an RNA recognition motif also have a putative prion domain. Meanwhile, several of these RNA-binding proteins have been independently identified as pathogenic in cases of ALS, FTLD-U, Alzheimer's disease, and Huntington's disease.
Role in neurodegenerative disease
The pathogenicity of prions and proteins with prion-like domains is hypothesized to arise from their self-templating ability and the resulting exponential growth of amyloid fibrils. The presence of amyloid fibrils in patients with degenerative diseases has been well documented. These amyloid fibrils are seen as the result of pathogenic proteins that self-propagate and form highly stable, non-functional aggregates. While this does not necessarily imply a causal relationship between amyloid and degenerative diseases, the toxicity of certain amyloid forms and the overproduction of amyloid in familial cases of degenerative disorders supports the idea that amyloid formation is generally toxic.TDP-43
Specifically, aggregation of TDP-43, an RNA-binding protein, has been found in ALS/MND patients, and mutations in the genes coding for these proteins have been identified in familial cases of ALS/MND. These mutations promote the misfolding of the proteins into a prion-like conformation. The misfolded form of TDP-43 forms cytoplasmic inclusions in affected neurons, and is found depleted in the nucleus. In addition to ALS/MND and FTLD-U, TDP-43 pathology is a feature of many cases of Alzheimer's disease, Parkinson's disease and Huntington's disease. The misfolding of TDP-43 is largely directed by its prion-like domain. This domain is inherently prone to misfolding, while pathological mutations in TDP-43 have been found to increase this propensity to misfold, explaining the presence of these mutations in familial cases of ALS/MND. As in yeast, the prion-like domain of TDP-43 has been shown to be both necessary and sufficient for protein misfolding and aggregation.RNPA2B1, RNPA1
Similarly, pathogenic mutations have been identified in the prion-like domains of heterogeneous nuclear riboproteins hnRNPA2B1 and hnRNPA1 in familial cases of muscle, brain, bone and motor neuron degeneration. The wild-type form of all of these proteins show a tendency to self-assemble into amyloid fibrils, while the pathogenic mutations exacerbate this behaviour and lead to excess accumulation.Alpha-synuclein
Both multiple system atrophy (MSA) and Parkinson's disease (PD) are associated with misfolded alpha-synuclein. In 2015, it was found that mice engineered to have a susceptible human version of alpha-synuclein become sick with MSA when injected in the brain with the brain homogenate of human MSA patients, but they do not get PD when injected with the brain homogenate of human PD patients. This suggests that the two diseases are different, with MSA being more transmissible. Misfolded alpha-synuclein from either Parkinson's disease or MSA can be detected by protein misfolding cyclic amplification (PMCA). The two forms, after PMCA, show different levels of fluorescence when bound to thioflavin T. This allows for distinguishing between the two diseases.Weaponization
Prions could theoretically be employed as a weaponized agent. With potential fatality rates of 100%, prions could be an effective bioweapon, sometimes called a "biochemical weapon", because a prion is a biochemical. An unfavorable aspect is prions' very long incubation periods. Persistent heavy exposure of prions to the intestine might shorten the overall onset. Another aspect of using prions in warfare is the difficulty of detection and decontamination.History
In the 18th and 19th centuries, exportation of sheep from Spain was observed to coincide with a disease called scrapie. This disease caused the affected animals to ''"lie down, bite at their feet and legs, rub their backs against posts, fail to thrive, stop feeding and finally become lame"''. The disease was also observed to have the long incubation period that is a key characteristic of transmissible spongiform encephalopathies (TSEs). Although the cause of scrapie was not known back then, it is probably the first transmissible spongiform encephalopathy to be recorded. In the 1950s, Carleton Gajdusek began research which eventually showed that kuru could be transmitted to chimpanzees by what was possibly a new infectious agent, work for which he eventually won the 1976Nobel Prize
The Nobel Prizes ( ; ; ) are awards administered by the Nobel Foundation and granted in accordance with the principle of "for the greatest benefit to humankind". The prizes were first awarded in 1901, marking the fifth anniversary of Alfred N ...
. During the 1960s, two London-based researchers, radiation biologist Tikvah Alper and biophysicist John Stanley Griffith, developed the hypothesis that the transmissible spongiform encephalopathies are caused by an infectious agent consisting solely of proteins. Earlier investigations by E.J. Field into scrapie and kuru had found evidence for the transfer of pathologically inert polysaccharides that only become infectious post-transfer, in the new host. Alper and Griffith wanted to account for the discovery that the mysterious infectious agent causing the diseases scrapie and Creutzfeldt–Jakob disease resisted ionizing radiation
Ionizing (ionising) radiation, including Radioactive decay, nuclear radiation, consists of subatomic particles or electromagnetic waves that have enough energy per individual photon or particle to ionization, ionize atoms or molecules by detaching ...
. Griffith proposed three ways in which a protein could be a pathogen
In biology, a pathogen (, "suffering", "passion" and , "producer of"), in the oldest and broadest sense, is any organism or agent that can produce disease. A pathogen may also be referred to as an infectious agent, or simply a Germ theory of d ...
.
In the first hypothesis
A hypothesis (: hypotheses) is a proposed explanation for a phenomenon. A scientific hypothesis must be based on observations and make a testable and reproducible prediction about reality, in a process beginning with an educated guess o ...
, he suggested that if the protein is the product of a normally suppressed gene
In biology, the word gene has two meanings. The Mendelian gene is a basic unit of heredity. The molecular gene is a sequence of nucleotides in DNA that is transcribed to produce a functional RNA. There are two types of molecular genes: protei ...
, and introducing the protein could induce the gene's expression, that is, wake the dormant gene up, then the result would be a process indistinguishable from replication, as the gene's expression would produce the protein, which would then wake the gene in other cells.
His second hypothesis forms the basis of the modern prion theory, and proposed that an abnormal form of a cellular protein can convert normal proteins of the same type into its abnormal form, thus leading to replication.
His third hypothesis proposed that the agent could be an antibody
An antibody (Ab) or immunoglobulin (Ig) is a large, Y-shaped protein belonging to the immunoglobulin superfamily which is used by the immune system to identify and neutralize antigens such as pathogenic bacteria, bacteria and viruses, includin ...
if the antibody was its own target antigen
In immunology, an antigen (Ag) is a molecule, moiety, foreign particulate matter, or an allergen, such as pollen, that can bind to a specific antibody or T-cell receptor. The presence of antigens in the body may trigger an immune response.
...
, as such an antibody would result in more and more antibody being produced against itself. However, Griffith acknowledged that this third hypothesis was unlikely to be true due to the lack of a detectable immune response.
Francis Crick recognized the potential significance of the Griffith protein-only hypothesis for scrapie propagation in the second edition of his " Central dogma of molecular biology" (1970): While asserting that the flow of sequence information from protein to protein, or from protein to RNA and DNA was "precluded", he noted that Griffith's hypothesis was a potential contradiction (although it was not so promoted by Griffith). The revised hypothesis was later formulated, in part, to accommodate reverse transcription (which both Howard Temin and David Baltimore discovered in 1970).
In 1982, Stanley B. Prusiner of the University of California, San Francisco, announced that his team had purified the hypothetical infectious protein, which did not appear to be present in healthy hosts, though they did not manage to isolate the protein until two years after Prusiner's announcement. The protein was named a prion, for "proteinacious infectious particle", derived from the words protein and infection. When the prion was discovered, Griffith's first hypothesis, that the protein was the product of a normally silent gene, was favored by many. It was subsequently discovered, however, that the same protein exists in normal hosts but in different form.
Following the discovery of the same protein in different form in uninfected individuals, the specific protein that the prion was composed of was named the prion protein (PrP), and Griffith's second hypothesis, that an abnormal form of a host protein can convert other proteins of the same type into its abnormal form, became the dominant theory. Prusiner was awarded the Nobel Prize in Physiology or Medicine in 1997 for his research into prions.
See also
* Bovine spongiform encephalopathy (BSE) * Diseases of abnormal polymerization * Mad cow crisis * Prion pseudoknot * Subviral agents * Tau protein * Beta amyloid *Proteinopathy
In medicine, proteinopathy ( 'pref''. protein -pathy 'suff''. disease proteinopathies ''pl''.; proteinopathic ''adj''), or proteopathy, protein conformational disorder, or protein misfolding disease, is a class of diseases in which certain prote ...
* Non-cellular life
Non-cellular life, also known as acellular life, is life that exists without a cellular structure for at least part of its life cycle. Historically, most definitions of life postulated that an organism must be composed of one or more cells, ...
References
External links
* CDC–World Health Organisation
– WHO information on prion diseases
The UK BSE Inquiry
nbsp;– Report of the UK public inquiry into BSE and variant CJD
UK Spongiform Encephalopathy Advisory Committee (SEAC)
* * {{Authority control Infectious diseases Amyloidosis