|
Fungal Prion
A fungal prion is a prion that infects hosts which are fungi. Fungal prions are naturally occurring proteins that can switch between multiple, structurally distinct conformations, at least one of which is self-propagating and transmissible to other prions. This transmission of protein state represents an epigenetic phenomenon where information is encoded in the protein structure itself, instead of in nucleic acids. Several prion-forming proteins have been identified in fungi, primarily in the yeast ''Saccharomyces cerevisiae''. These fungal prions are generally considered benign, and in some cases even confer a selectable advantage to the organism. Fungal prions have provided a model for the understanding of disease-forming mammalian prions. Study of fungal prions has led to a characterisation of the sequence features and mechanisms that enable prion domains to switch between functional and amyloid-forming states. Sequence features Prions are formed by portable, transmissible pri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prion
A prion () is a Proteinopathy, misfolded protein that induces misfolding in normal variants of the same protein, leading to cellular death. Prions are responsible for prion diseases, known as transmissible spongiform encephalopathy (TSEs), which are fatal and transmissible neurodegenerative diseases affecting both humans and animals. These proteins can misfold sporadically, due to genetic mutations, or by exposure to an already misfolded protein, leading to an abnormal Protein tertiary structure, three-dimensional structure that can propagate misfolding in other proteins. The term ''prion'' comes from "proteinaceous infectious particle". Unlike other infectious agents such as viruses, bacteria, and fungi, prions do not contain nucleic acids (DNA or RNA). Prions are mainly twisted Protein isoform, isoforms of the major prion protein (PrP), a naturally occurring protein with an uncertain function. They are the hypothesized cause of various transmissible spongiform encephalopath ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adenine
Adenine (, ) (nucleoside#List of nucleosides and corresponding nucleobases, symbol A or Ade) is a purine nucleotide base that is found in DNA, RNA, and Adenosine triphosphate, ATP. Usually a white crystalline subtance. The shape of adenine is complementary and pairs to either thymine in DNA or uracil in RNA. In cells adenine, as an independent molecule, is rare. It is almost always covalent bond, covalently bound to become a part of a larger biomolecule. Adenine has a central role in cellular respiration. It is part of adenosine triphosphate which provides the energy that drives and supports most activities in living cell (biology), cells, such as Protein biosynthesis, protein synthesis, chemical synthesis, muscle contraction, and nerve impulse propagation. In respiration it also participates as part of the cofactor (biochemistry), cofactors nicotinamide adenine dinucleotide, flavin adenine dinucleotide, and Coenzyme A. It is also part of adenosine, adenosine monophosphate, cy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Population Genetic
Population genetics is a subfield of genetics that deals with genetic differences within and among populations, and is a part of evolutionary biology. Studies in this branch of biology examine such phenomena as adaptation, speciation, and population structure. Population genetics was a vital ingredient in the emergence of the modern evolutionary synthesis. Its primary founders were Sewall Wright, J. B. S. Haldane and Ronald Fisher, who also laid the foundations for the related discipline of quantitative genetics. Traditionally a highly mathematical discipline, modern population genetics encompasses theoretical, laboratory, and field work. Population genetic models are used both for statistical inference from DNA sequence data and for proof/disproof of concept. What sets population genetics apart from newer, more phenotypic approaches to modelling evolution, such as evolutionary game theory and adaptive dynamics, is its emphasis on such genetic phenomena as dominance, epistas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Evolutionary Capacitance
Evolutionary capacitance is the storage and release of variation, just as electric capacitors store and release charge. Living systems are robust to mutations. This means that living systems accumulate genetic variation without the variation having a phenotypic effect. But when the system is disturbed (perhaps by stress), robustness breaks down, and the variation has phenotypic effects and is subject to the full force of natural selection. An evolutionary capacitor is a molecular switch mechanism that can "toggle" genetic variation between hidden and revealed states. If some subset of newly revealed variation is adaptive, it becomes fixed by genetic assimilation. After that, the rest of variation, most of which is presumably deleterious, can be switched off, leaving the population with a newly evolved advantageous trait, but no long-term handicap. For evolutionary capacitance to increase evolvability in this way, the switching rate should not be faster than the timescale of geneti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Natural Selection
Natural selection is the differential survival and reproduction of individuals due to differences in phenotype. It is a key mechanism of evolution, the change in the Heredity, heritable traits characteristic of a population over generations. Charles Darwin popularised the term "natural selection", contrasting it with selective breeding, artificial selection, which is intentional, whereas natural selection is not. Genetic diversity, Variation of traits, both Genotype, genotypic and phenotypic, exists within all populations of organisms. However, some traits are more likely to facilitate survival and reproductive success. Thus, these traits are passed the next generation. These traits can also become more Allele frequency, common within a population if the environment that favours these traits remains fixed. If new traits become more favoured due to changes in a specific Ecological niche, niche, microevolution occurs. If new traits become more favoured due to changes in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
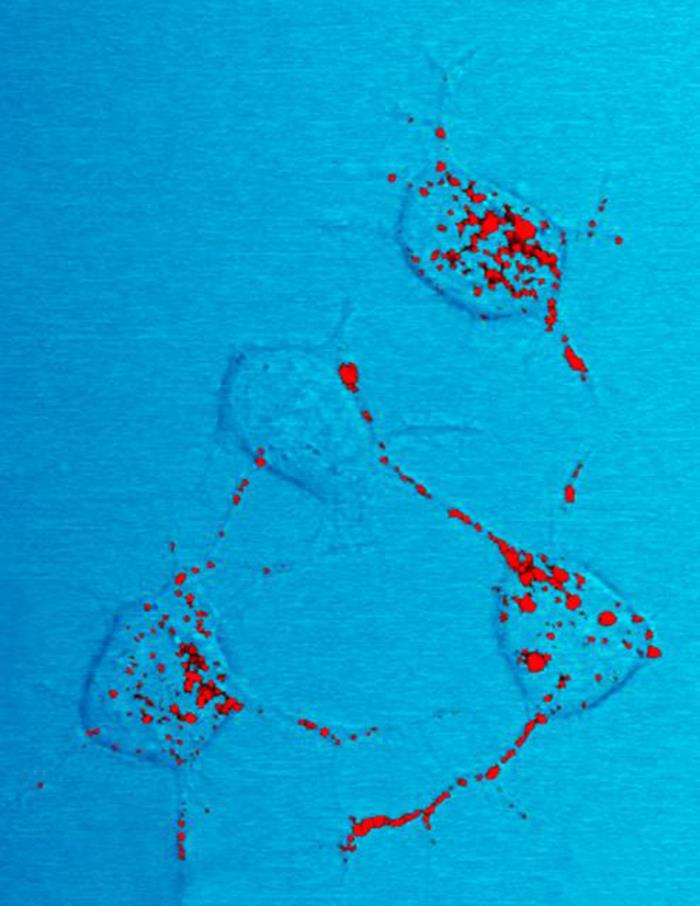
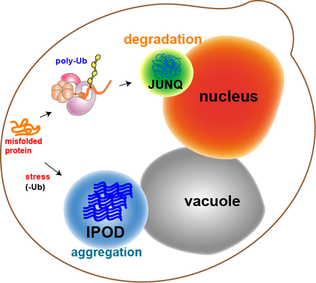
JUNQ And IPOD
JUNQ and IPOD are types of cytosolic protein inclusion bodies in eukaryotes. Neurodegenerative diseases, such as Parkinson's, Alzheimer's, and Huntington's, are associated and correlated with protein aggregation and accumulation of misfolded proteins in inclusion bodies. For many years, protein aggregation was considered a random process by which misfolded proteins stick to each other to form inclusions (imagine a bundle of hairs haphazardly piling up in a corner of a room). Moreover, protein aggregates were thought to be toxic agents and the cause for neuronal dysfunction and death. However, recent studies, using advanced methods (i.e. fluorescence microscopy), show that protein aggregation may actually be a tightly regulated, organized process, by which the cell protects itself from toxic proteins by sequestration to inclusion bodies. In 2008, Daniel Kaganovich working in the Frydman lab showed that eukaryotic cells sort misfolded proteins into two distinct inclusion bodies in a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PRNP
The major prion protein (PrP) is encoded in the human body by the ''PRNP'' gene also known as CD230 (cluster of differentiation 230). Expression of the protein is most predominant in the nervous system but occurs in many other tissues throughout the body. The protein can exist in multiple isoforms: the normal PrPC form, and the protease-resistant form designated PrPRes such as the disease-causing PrPSc (scrapie) and an isoform located in mitochondria. The misfolded version PrPSc is associated with a variety of cognitive disorders and neurodegenerative diseases such as in animals: ovine scrapie, bovine spongiform encephalopathy (BSE, mad cow disease), feline spongiform encephalopathy, transmissible mink encephalopathy (TME), exotic ungulate encephalopathy, chronic wasting disease (CWD) which affects deer; and in humans: Creutzfeldt–Jakob disease (CJD), fatal familial insomnia (FFI), Gerstmann–Sträussler–Scheinker syndrome (GSS), kuru, and variant Creutzfeldt–J ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chaperone (protein)
In molecular biology, molecular chaperones are proteins that assist the conformational folding or unfolding of large proteins or macromolecular protein complexes. There are a number of classes of molecular chaperones, all of which function to assist large proteins in proper protein folding during or after synthesis, and after partial denaturation. Chaperones are also involved in the translocation of proteins for proteolysis. The first molecular chaperones discovered were a type of assembly chaperones which assist in the assembly of nucleosomes from folded histones and DNA. One major function of molecular chaperones is to prevent the aggregation of misfolded proteins, thus many chaperone proteins are classified as heat shock proteins, as the tendency for protein aggregation is increased by heat stress. The majority of molecular chaperones do not convey any steric information for protein folding, and instead assist in protein folding by binding to and stabilizing folding intermedi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amyloid
Amyloids are aggregates of proteins characterised by a fibrillar morphology of typically 7–13 nm in diameter, a β-sheet secondary structure (known as cross-β) and ability to be stained by particular dyes, such as Congo red. In the human body, amyloids have been linked to the development of various diseases. Pathogenic amyloids form when previously healthy proteins lose their normal structure and physiological functions ( misfolding) and form fibrous deposits within and around cells. These protein misfolding and deposition processes disrupt the healthy function of tissues and organs. Such amyloids have been associated with (but not necessarily as the cause of) more than 50 human diseases, known as amyloidosis, and may play a role in some neurodegenerative diseases. Some of these diseases are mainly sporadic and only a few cases are familial. Others are only familial. Some result from medical treatment. Prions are an infectious form of amyloids that can act as a templa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Synthesis
Protein biosynthesis, or protein synthesis, is a core biological process, occurring inside cells, balancing the loss of cellular proteins (via degradation or export) through the production of new proteins. Proteins perform a number of critical functions as enzymes, structural proteins or hormones. Protein synthesis is a very similar process for both prokaryotes and eukaryotes but there are some distinct differences. Protein synthesis can be divided broadly into two phases: transcription and translation. During transcription, a section of DNA encoding a protein, known as a gene, is converted into a molecule called messenger RNA (mRNA). This conversion is carried out by enzymes, known as RNA polymerases, in the nucleus of the cell. In eukaryotes, this mRNA is initially produced in a premature form (pre-mRNA) which undergoes post-transcriptional modifications to produce mature mRNA. The mature mRNA is exported from the cell nucleus via nuclear pores to the cytoplasm of the c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ure2p
Ure2p is a yeast protein that represses transcription of genes involved in nitrogen catabolism. It specifically regulates the utilization of poor nitrogen sources in the presence of preferred nutrients such as ammonia or glutamine. Ure2p is one of the few yeast proteins that are known to be prions. At low frequency the protein adopts an alternative conformation that is auto-catalytic and self-propagating. Yeast cells that carry the protein in the prion conformation are designated as RE3 Autocatalytic conversion of Ure2p into the inactive prion form of the protein Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ... results in a loss of repression of nitrogen catabolic genes. References Prions {{Protein-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sup35p
Sup35p is the ''Saccharomyces cerevisiae'' (a yeast) eukaryotic translation release factor. More specifically, it is the yeast eukaryotic release factor 3 (eRF3), which forms the translation termination complex with eRF1 ( Sup45p in yeast). This complex recognizes and catalyzes the release of the nascent polypeptide chain when the ribosome encounters a stop codon. While eRF1 recognizes stop codons, eRF3 facilitates the release of the polypeptide chain through GTP hydrolysis. Partial loss of function results in nonsense suppression, in which stop codons are ignored and proteins are abnormally synthesized with carboxyl terminal extensions. Complete loss of function is fatal. History Sup35p was shown to propagate in a prion form in 1994 by Reed Wickner. For this reason it is an intensely studied protein. When yeast cells harbor Sup35p in the prion state the resulting phenotype is known as SI+ In SI+cells Sup35p exists in an amyloid state that can be propagated and passed to daug ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |