|
Dimethylammonium
Dimethylamine is an organic compound with the formula (CH3)2NH. This secondary amine is a colorless, flammable gas with an ammonia-like odor. Dimethylamine is commonly encountered commercially as a solution in water at concentrations up to around 40%. An estimated 270,000 tons were produced in 2005. Structure and synthesis The molecule consists of a nitrogen atom with two methyl substituents and one hydrogen. Dimethylamine is a weak base and the pKa of the ammonium CH3--CH3 is 10.73, a value above methylamine (10.64) and trimethylamine (9.79). Dimethylamine reacts with acids to form salts, such as dimethylamine hydrochloride, an odorless white solid with a melting point of 171.5 °C. Dimethylamine is produced by catalytic reaction of methanol and ammonia at elevated temperatures and high pressure: : Natural occurrence Dimethylamine is found quite widely distributed in animals and plants, and is present in many foods at the level of a few mg/kg. Uses Dimethylamine is a precu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium
Ammonium is a modified form of ammonia that has an extra hydrogen atom. It is a positively charged (cationic) polyatomic ion, molecular ion with the chemical formula or . It is formed by the protonation, addition of a proton (a hydrogen nucleus) to ammonia (). Ammonium is also a general name for positively charged (protonated) substituted amines and quaternary ammonium cations (), where one or more hydrogen atoms are replaced by Organic compound, organic or other groups (indicated by R). Not only is ammonium a source of nitrogen and a key metabolite for many living organisms, but it is an integral part of the global nitrogen cycle. As such, human impact in recent years could have an effect on the biological communities that depend on it. Acid–base properties The ammonium ion is generated when ammonia, a weak base, reacts with Brønsted–Lowry acid–base theory, Brønsted acids (proton donors): : The ammonium ion is mildly acidic, reacting with Brønsted bases to return ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylamine
Methylamine, also known as methanamine, is an organic compound with a formula of . This colorless gas is a derivative of ammonia, but with one hydrogen atom being replaced by a methyl group. It is the simplest primary amine. Methylamine is sold as a solution in methanol, ethanol, tetrahydrofuran, or water, or as the anhydrous gas in pressurized metal containers. Industrially, methylamine is transported in its anhydrous form in pressurized railcars and tank trailers. It has a strong odor similar to rotten fish. Methylamine is used as a building block for the synthesis of numerous other commercially available compounds. Industrial production Methylamine has been produced industrially since the 1920s (originally by Commercial Solvents Corporation for dehairing of animal skins). This was made possible by and his wife Eugenia who discovered amination of alcohols, including methanol, on alumina or kaolin catalyst after WWI, filed two patent applications in 1919 and published an a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Agrichemical
An agrochemical or agrichemical, a contraction of ''agricultural chemical'', is a chemical product used in industrial agriculture. Agrichemical typically refers to biocides (pesticides including insecticides, herbicides, fungicides and nematicides) alongside synthetic fertilizers. It may also include hormones and other chemical growth agents. Though the application of mineral fertilizers and pesticidal chemicals has a long history, the majority of agricultural chemicals were developed from the 19th century, and their use were expanded significantly during the Green Revolution and the late 20th century. Agriculture that uses these chemicals is frequently called conventional agriculture. Agrochemicals are counted among speciality chemicals. Most agrochemicals are products of the petrochemical industry, where chemicals are derivatives of fossil fuels. The production and use of agrochemicals contribute substantially to climate change, both through direct emissions during pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethylacetamide
Dimethylacetamide (DMAc or DMA) is the organic compound with the formula CH3C(O)N(CH3)2. This colorless, water-miscible, high-boiling liquid is commonly used as a polar solvent in organic synthesis. DMA is miscible with most other solvents, although it is poorly soluble in aliphatic hydrocarbons. Synthesis and production DMA is prepared commercially by the reaction of dimethylamine with acetic anhydride or acetic acid. Dehydration of the salt of dimethylamine and acetic acid also furnishes this compound: : CH3CO2H·HN(CH3)2 → H2O + CH3CON(CH3)2 Dimethylacetamide can also be produced by the reaction of dimethylamine with methyl acetate. : The separation and purification of the product is carried out by multistage distillation in rectification columns. DMA is obtained with essentially quantitive (99%) yield referred to methyl acetate. Reactions and applications The chemical reactions of dimethylacetamide are typical of ''N'',''N''-disubstituted amides. Hydrolysis of the acyl- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethylformamide
Dimethylformamide, DMF is an organic compound with the chemical formula . Its structure is . Commonly abbreviated as DMF (although this initialism is sometimes used for 2,5-dimethylfuran, dimethylfuran, or dimethyl fumarate), this colourless liquid is Miscibility, miscible with Water (molecule), water and the majority of organic liquids. DMF is a common solvent for chemical reactions. Dimethylformamide is odorless, but chemical purity, technical-grade or degraded samples often have a fishy smell due to impurity of dimethylamine. Dimethylamine degradation impurities can be removed by Sparging (chemistry), sparging samples with an inert gas such as argon or by sonication, sonicating the samples under reduced pressure. As its name indicates, it is structurally related to formamide, having two methyl groups in the place of the two hydrogens. DMF is a polar molecule, polar (hydrophilic) aprotic solvent with a high boiling point. It facilitates reactions that follow polar mechanisms, such ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethylene Oxide
Ethylene oxide is an organic compound with the chemical formula, formula . It is a cyclic ether and the simplest epoxide: a three-membered ring (chemistry), ring consisting of one oxygen atom and two carbon atoms. Ethylene oxide is a colorless and flammable gas with a faintly sweet odor. Because it is a strained ring, ethylene oxide easily participates in a number of addition reactions that result in ring-opening. Ethylene oxide is isomeric with acetaldehyde and with vinyl alcohol. Ethylene oxide is industrially produced by oxidation of ethylene in the presence of a silver catalyst. The reactivity that is responsible for many of ethylene oxide's hazards also makes it useful. Although too dangerous for direct household use and generally unfamiliar to consumers, ethylene oxide is used for making many consumer products as well as non-consumer chemicals and intermediates. These products include detergents, thickeners, solvents, plastics, and various organic chemicals such as ethylen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethylaminoethoxyethanol
2- -(Dimethylamino)ethoxythanol is an organic compound with the molecular formula C6H15NO2 and is a liquid at room temperature. Dimethylaminoethoxyethanol is polyfunctional, having a tertiary amine, ether and hydroxyl functionality. Like other organic amines, it acts as a weak base. Manufacture Dimethylaminoethoxyethanol is manufactured by reacting dimethylamine and ethylene oxide. Other methods are also available producing streams rich in the substance which then need to be further purified. Uses As dimethylaminoethoxyethanol is weakly basic, it has been studied as a method of absorbing Greenhouse gases and in particular carbon dioxide. Dimethylaminoethoxyethanol is used extensively in surfactants which have also been evaluated as corrosion inhibitors. Surfactants prepared are usually cationic and may also be used as a biocide. This is particularly important for oilfield applications against Sulfate-reducing microorganism Sulfate-reducing microorganisms (SRM) or sulfate-reduc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rubber
Rubber, also called India rubber, latex, Amazonian rubber, ''caucho'', or ''caoutchouc'', as initially produced, consists of polymers of the organic compound isoprene, with minor impurities of other organic compounds. Types of polyisoprene that are used as natural rubbers are classified as elastomers. Currently, rubber is harvested mainly in the form of the latex from the Hevea brasiliensis, Pará rubber tree (''Hevea brasiliensis'') or others. The latex is a sticky, milky and white colloid drawn off by making incisions in the bark and collecting the fluid in vessels in a process called "tapping". Manufacturers refine this latex into the rubber that is ready for commercial processing. Natural rubber is used extensively in many applications and products, either alone or in combination with other materials. In most of its useful forms, it has a large stretch ratio and high resilience and also is buoyant and water-proof. Industrial demand for rubber-like materials began to out ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur Vulcanization
Sulfur vulcanization is a chemical process for converting natural rubber or related polymers into materials of varying hardness, elasticity, and mechanical durability by heating them with sulfur or sulfur-containing compounds. Sulfur forms cross-linking bridges between sections of polymer chains which affects the mechanical properties. Many products are made with vulcanized rubber, including tires, shoe soles, hoses, and conveyor belts. The term is derived from Vulcan (mythology), Vulcan, the Roman god of fire. The main polymers subjected to sulfur vulcanization are polyisoprene (natural rubber, NR), polybutadiene rubber (BR) and styrene-butadiene rubber (SBR), and ethylene propylene diene monomer rubber (EPDM rubber). All of these materials contain allyl, alkene groups adjacent to methylene groups. Other specialty rubbers may also be vulcanized, such as nitrile rubber (NBR) and butyl rubber (IIR). Vulcanization, in common with the curing of other thermosetting polymers, is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
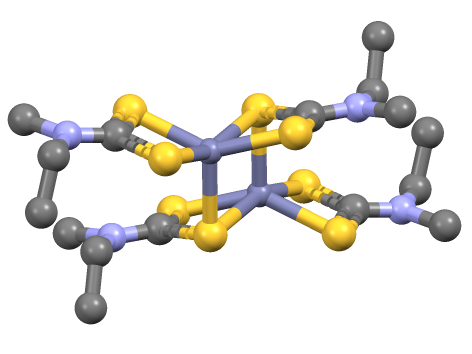
Zinc Bis(dimethyldithiocarbamate)
Zinc dimethyldithiocarbamate is a coordination complex of zinc with dimethyldithiocarbamate. It is a pale yellow solid that is used as a fungicide, the sulfur vulcanization of rubber, and other industrial applications. Applications Known as ziram in agriculture, it was introduced in the United States in 1960 as a broad-spectrum fungicide. It was used to address scab on apples and pears, leaf curl in peaches, and anthracnose and blight in tomatoes. In 1981, additional uses for ziram were approved, including the prevention of leaf blight and scab on almonds, shot-hole in apricots, brown rot and leaf spot in cherries, and scab and anthracnose in pecans. Ziram also began to be used on residential ornaments as a bird and mammal repellent. As a protectant fungicide, it is active on the plant’s surface where it forms a chemical barrier between the plant and a fungus. A protectant fungicide is not absorbed into the plant and must be applied prior to infection. Ziram can either be direc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dithiocarbamate
In organic chemistry, a dithiocarbamate is a chemical compound with the general formula . It contains the functional group with the Chemical structure, structure . It is the analog of a carbamate in which both oxygen atoms are replaced by sulfur atoms (when only one oxygen is replaced the result is thiocarbamate). Dithiocarbamate also refers to the dithiocarbamate ion and its salts. A common example is sodium diethyldithiocarbamate . Dithiocarbamates and their derivatives are widely used in the vulcanization of rubber. Formation Many secondary amines react with carbon disulfide and sodium hydroxide to form dithiocarbamate salts: : Ammonia reacts with carbon disulfide, similarly, to give ammonium dithiocarbamate: : Dithiocarbamate salts are pale colored solids that are soluble in water and Solvent, polar organic solvents. Dithiocarbamic acid A primary amine and carbon disulfide react to give a dithiocarbamic acid: : In the presence of diimides or pyridine, these acids convert t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Disulfide
Carbon disulfide (also spelled as carbon disulphide) is an inorganic compound with the chemical formula and structure . It is also considered as the anhydride of thiocarbonic acid. It is a colorless, flammable, neurotoxic liquid that is used as a building block in organic synthesis. Pure carbon disulfide has a pleasant, ether- or chloroform-like odor, but commercial samples are usually yellowish and are typically contaminated with foul-smelling impurities.. History In 1796, the German chemist Wilhelm August Lampadius (1772–1842) first prepared carbon disulfide by heating pyrite with moist charcoal. He called it "liquid sulfur" (''flüssig Schwefel''). The composition of carbon disulfide was finally determined in 1813 by the team of the Swedish chemist Jöns Jacob Berzelius (1779–1848) and the Swiss-British chemist Alexander Marcet (1770–1822). Their analysis was consistent with an empirical formula of CS2. Occurrence, manufacture, properties Small amounts of carbon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |