|
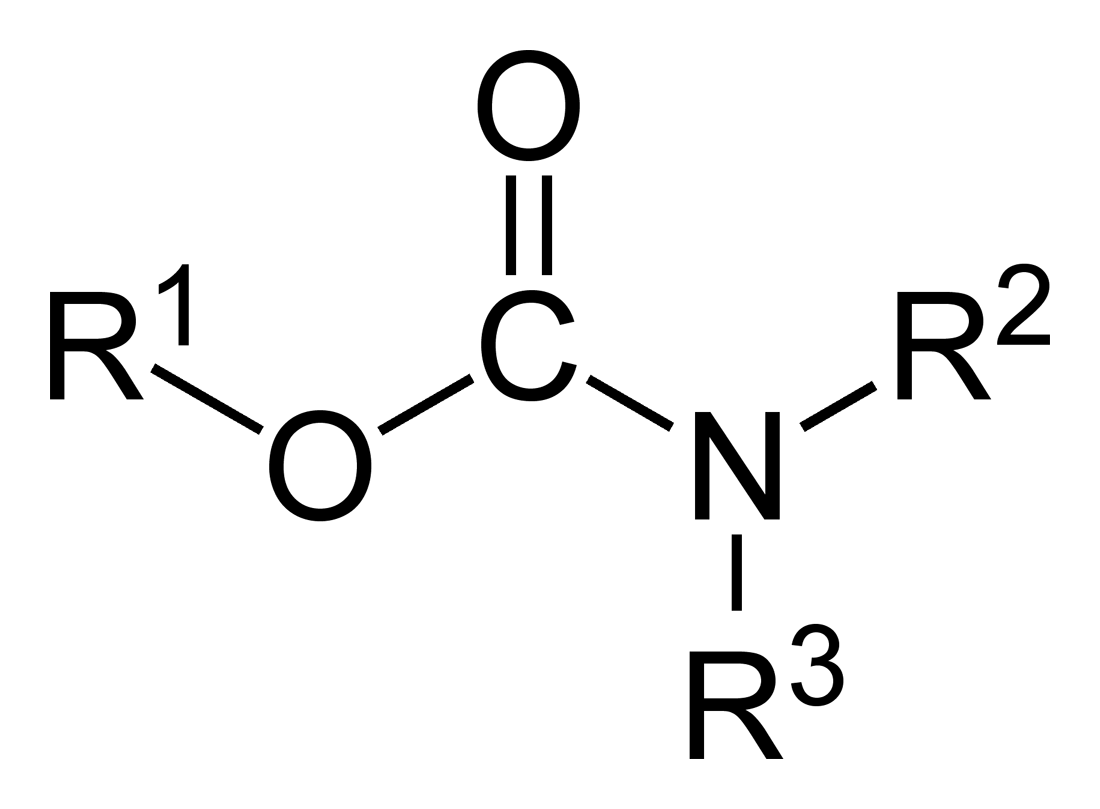
Dithiocarbamate
In organic chemistry, a dithiocarbamate is a chemical compound with the general formula . It contains the functional group with the Chemical structure, structure . It is the analog of a carbamate in which both oxygen atoms are replaced by sulfur atoms (when only one oxygen is replaced the result is thiocarbamate). Dithiocarbamate also refers to the dithiocarbamate ion and its salts. A common example is sodium diethyldithiocarbamate . Dithiocarbamates and their derivatives are widely used in the vulcanization of rubber. Formation Many secondary amines react with carbon disulfide and sodium hydroxide to form dithiocarbamate salts: : Ammonia reacts with carbon disulfide, similarly, to give ammonium dithiocarbamate: : Dithiocarbamate salts are pale colored solids that are soluble in water and Solvent, polar organic solvents. Dithiocarbamic acid A primary amine and carbon disulfide react to give a dithiocarbamic acid: : In the presence of diimides or pyridine, these acids convert t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
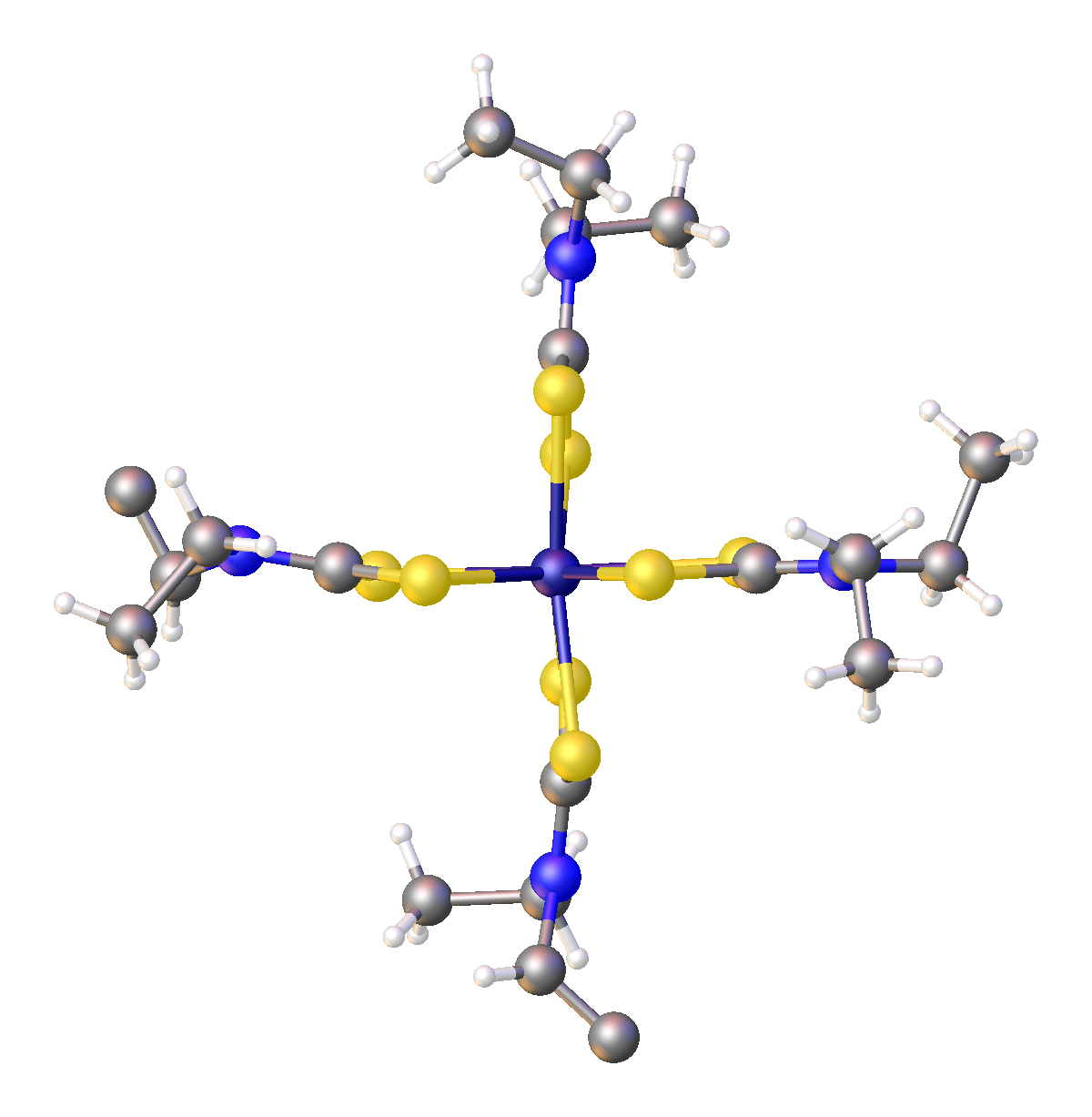
Transition Metal Dithiocarbamate Complexes
193px, Structure of iron tris(diethyldithiocarbamate). Transition metal dithiocarbamate complexes are coordination complexes containing one or more dithiocarbamate ligand, which are typically abbreviated R2dtc−. Many complexes are known. Several homoleptic derivatives have the formula M(R2dtc)n where n = 2 and 3. Ligand characteristics Dithiocarbamate anions are bidentate ligands that are classified as L-X ligand in the Covalent bond classification method. In the usual electron counting method, they are three-electron ligands. With respect to HSAB theory, they are classified as soft. Because of the pi-donor properties of the amino substituent, the two sulfur centers show enhanced basicity relative to dithiocarboxylates. This situation is represented by the zwitterionic resonance structure that depicts a positive charge on N and negative charges on both sulfurs. This N to C pi-bonding results in partial double bond character for the C-N bond. Consequently, barriers to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dithiocarbamate
In organic chemistry, a dithiocarbamate is a chemical compound with the general formula . It contains the functional group with the Chemical structure, structure . It is the analog of a carbamate in which both oxygen atoms are replaced by sulfur atoms (when only one oxygen is replaced the result is thiocarbamate). Dithiocarbamate also refers to the dithiocarbamate ion and its salts. A common example is sodium diethyldithiocarbamate . Dithiocarbamates and their derivatives are widely used in the vulcanization of rubber. Formation Many secondary amines react with carbon disulfide and sodium hydroxide to form dithiocarbamate salts: : Ammonia reacts with carbon disulfide, similarly, to give ammonium dithiocarbamate: : Dithiocarbamate salts are pale colored solids that are soluble in water and Solvent, polar organic solvents. Dithiocarbamic acid A primary amine and carbon disulfide react to give a dithiocarbamic acid: : In the presence of diimides or pyridine, these acids convert t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Diethyldithiocarbamate
Sodium diethyldithiocarbamate is the organosulfur compound with the formula . It is a pale yellow, water soluble salt. Preparation Sodium diethyldithiocarbamate typically crystallizes from water as the trihydrate . The anhydrous salt and the trihydrate are often used interchangeably. Sodium diethyldithiocarbamate is obtained by treating carbon disulfide with diethylamine in the presence of sodium hydroxide: :CS2 + HN(C2H5)2 + NaOH → NaS2CN(C2H5)2 + H2O Other dithiocarbamates can be prepared similarly from secondary amines and carbon disulfide. They are used as chelating agents for transition metal ions and as precursors to herbicides and vulcanization reagents. Reactions Oxidation of sodium diethyldithiocarbamate gives the disulfide, also called a thiuram disulfide (Et = ethyl): : Dithiocarbamates are nucleophiles and thus can be alkylated. Even dichloromethane suffices: : Diethyldithiocarbamate reacts with many metal salts to give transition metal dithiocar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiuram Disulfide
Thiuram disulfides are a class of organosulfur compounds with the formula (R2NCSS)2. Many examples are known, but popular ones include R = Me and R = Et. They are disulfides obtained by oxidation of the dithiocarbamates. These compounds are used in sulfur vulcanization of rubber as well as in the manufacture of pesticides and drugs. They are typically white or pale yellow solids that are soluble in organic solvents. Preparation, structure, reactions Thiuram disulfides are prepared by oxidizing the salts of the corresponding dithiocarbamates (e.g. sodium diethyldithiocarbamate). Typical oxidants employed include chlorine and hydrogen peroxide: : Thiuram disulfides react with Grignard reagents to give esters of dithiocarbamic acid, as in the preparation of methyl dimethyldithiocarbamate: : The compounds feature planar dithiocarbamate subunits and are linked by an S−S bond of 2.00 Å. The C(S)−N bond is short (1.33 Å), indicative of multiple bonding. The dihe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Maneb
Maneb (manganese ethylene-bis-dithiocarbamate) is a fungicide and a polymeric complex of manganese with the ethylene bis (dithiocarbamate) anionic ligand. Health effects Exposure to maneb can occur when breathed in; it can irritate the eyes, nose, and throat as well as cause headache, fatigue, nervousness, dizziness, seizures and even unconsciousness. Prolonged or long-term exposure may interfere with the function of the thyroid. Exposure to maneb is also shown to induce a Parkinson's disease like neurotoxicity in mice. It is still challenged whether maneb, along with Paraquat, is an environmental risk factor for Parkinson's disease. Production Manganese(II) ethylenebis(dithiocarbamate) of low ethylenethiourea (ETU) content is prepared by mixing disodium ethylenebis (dithiocarbamate) with formaldehyde in aqueous medium then mixing a water-soluble manganese(II) salt to precipitate the maneb. The product can be further formulated with a metal salt and also with paraforma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Dimethyldithiocarbamate
Methyl dimethyldithiocarbamate is the organosulfur compound with the formula . It is the one of simplest dithiocarbamic esters. It is a white volatile solid that is poorly soluble in water but soluble in many organic solvents. It was once used as a pesticide. Methyl dimethyldithiocarbamate can be prepared by methylation of salts of dimethyldithiocarbamate: : It can also be prepared by the reaction of a tetramethylthiuram disulfide Thiuram disulfides are a class of organosulfur compounds with the formula (R2NCSS)2. Many examples are known, but popular ones include R = Me and R = Et. They are disulfides obtained by oxidation of the dithiocarbamates. These compounds are use ... with methyl Grignard reagents: :{{chem2, CH3)2NC(S)S + CH3MgBr → (CH3)2NC(S)SCH3 + (CH3)2NCS2MgBr References Dithiocarbamates Dimethylamino compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Disulfide
Carbon disulfide (also spelled as carbon disulphide) is an inorganic compound with the chemical formula and structure . It is also considered as the anhydride of thiocarbonic acid. It is a colorless, flammable, neurotoxic liquid that is used as a building block in organic synthesis. Pure carbon disulfide has a pleasant, ether- or chloroform-like odor, but commercial samples are usually yellowish and are typically contaminated with foul-smelling impurities.. History In 1796, the German chemist Wilhelm August Lampadius (1772–1842) first prepared carbon disulfide by heating pyrite with moist charcoal. He called it "liquid sulfur" (''flüssig Schwefel''). The composition of carbon disulfide was finally determined in 1813 by the team of the Swedish chemist Jöns Jacob Berzelius (1779–1848) and the Swiss-British chemist Alexander Marcet (1770–1822). Their analysis was consistent with an empirical formula of CS2. Occurrence, manufacture, properties Small amounts of carbon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbamate
In organic chemistry, a carbamate is a category of organic compounds with the general Chemical formula, formula and Chemical structure, structure , which are formally Derivative (chemistry), derived from carbamic acid (). The term includes organic compounds (e.g., the ester ethyl carbamate), formally obtained by replacing one or more of the hydrogen atoms by other organic functional groups; as well as salt (chemistry), salts with the carbamate anion (e.g. ammonium carbamate). Polymers whose repeat units are joined by carbamate like groups are an important family of plastics, the polyurethanes. See for clarification. Properties While carbamic acids are unstable, many carbamate esters and salt (chemistry), salts are stable and well known. Equilibrium with carbonate and bicarbonate In water solutions, the carbamate anion slowly equilibrates with the ammonium cation and the carbonate or bicarbonate anions: : : Calcium carbamate is soluble in water, whereas calcium carbona ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isothiocyanate
In organic chemistry, isothiocyanate is a functional group as found in compounds with the formula . Isothiocyanates are the more common isomers of thiocyanates, which have the formula . Occurrence Many isothiocyanates from plants are produced by enzymatic conversion of metabolites called glucosinolates. A prominent natural isothiocyanate is allyl isothiocyanate, also known as mustard oils. Cruciferous vegetables, such as bok choy, broccoli, cabbage, cauliflower, kale, and others, are rich sources of glucosinolate precursors of isothiocyanates. Structure The and distances are 117 and 158 pm. By contrast, in methyl thiocyanate, and distances are 116 and 176 pm. Typical bond angles for in aryl isothiocyanates are near 165°. Again, the thiocyanate isomers are quite different with angle near 100°. In both isomers the angle approaches 180°. Synthesis Allyl thiocyanate isomerizes to the isothiocyanate: : Isothiocyanates can be prepared by treating organ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Xanthate
A xanthate is a Salt (chemistry), salt or ester of a xanthic acid. The formula of the salt of xanthic acid is (where R is organyl group and M is usually Sodium, Na or Potassium, K). Xanthate also refers to the anion . The formula of a xanthic acid is , such as ethyl xanthic acid, while the formula of a xanthate ester is , where R and R' are organyl groups. The salts of xanthates are sometimes called ''O''-organyl dithioates. The esters of xanthic acid are sometimes called ''O'',''S''-diorganyl esters of Dithiocarbonate, dithiocarbonic acid. The name ''xanthate'' is derived from Ancient Greek (''xanthos'') meaning 'yellowish' or 'golden', and indeed most xanthate salts are yellow. They were discovered and named in 1823 by Danish chemist William Christopher Zeise. These organosulfur compounds are important in two areas: the production of cellophane and related polymers from cellulose and (in mining) for extraction of certain sulphide bearing ores. They are also versatile intermedi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Manganese
Manganese is a chemical element; it has Symbol (chemistry), symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese was first isolated in the 1770s. It is a transition metal with a multifaceted array of industrial alloy uses, particularly in stainless steels. It improves strength, workability, and resistance to wear. Manganese oxide is used as an oxidising agent, as a rubber additive, and in glass making, fertilisers, and ceramics. Manganese sulfate can be used as a fungicide. Manganese is also an essential human dietary element, important in macronutrient metabolism, bone formation, and free radical defense systems. It is a critical component in dozens of proteins and enzymes. It is found mostly in the bones, but also the liver, kidneys, and brain. In the human brain, the manganese is bound to manganese metalloproteins, most notably glutamine synthetase in astrocytes. Manganese is commonly found in labo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |