|
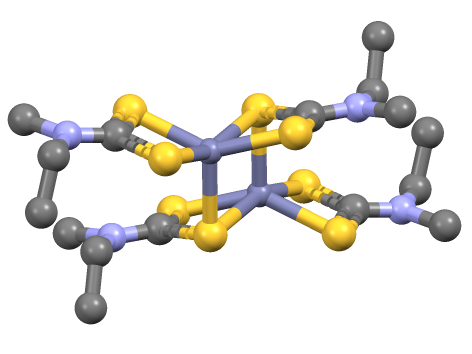
Zinc Bis(dimethyldithiocarbamate)
Zinc dimethyldithiocarbamate is a coordination complex of zinc with dimethyldithiocarbamate. It is a pale yellow solid that is used as a fungicide, the sulfur vulcanization of rubber, and other industrial applications. Applications Known as ziram in agriculture, it was introduced in the United States in 1960 as a broad-spectrum fungicide. It was used to address scab on apples and pears, leaf curl in peaches, and anthracnose and blight in tomatoes. In 1981, additional uses for ziram were approved, including the prevention of leaf blight and scab on almonds, shot-hole in apricots, brown rot and leaf spot in cherries, and scab and anthracnose in pecans. Ziram also began to be used on residential ornaments as a bird and mammal repellent. As a protectant fungicide, it is active on the plant’s surface where it forms a chemical barrier between the plant and a fungus. A protectant fungicide is not absorbed into the plant and must be applied prior to infection. Ziram can either be direc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coordination Complex
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of chemical bond, bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many metal-containing chemical compound, compounds, especially those that include transition metals (elements like titanium that belong to the periodic table's d-block), are coordination complexes. Nomenclature and terminology Coordination complexes are so pervasive that their structures and reactions are described in many ways, sometimes confusingly. The atom within a ligand that is bonded to the central metal atom or ion is called the donor atom. In a typical complex, a metal ion is bonded to several donor atoms, which can be the same or different. A Ligand#Polydentate and polyhapto ligand motifs and nomenclature, polydentate (multiple bonded) ligand is a molecule or ion that bonds to the central atom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bird
Birds are a group of warm-blooded vertebrates constituting the class (biology), class Aves (), characterised by feathers, toothless beaked jaws, the Oviparity, laying of Eggshell, hard-shelled eggs, a high Metabolism, metabolic rate, a four-chambered heart, and a strong yet lightweight Bird skeleton, skeleton. Birds live worldwide and range in size from the bee hummingbird to the common ostrich. There are over 11,000 living species and they are split into 44 Order (biology), orders. More than half are passerine or "perching" birds. Birds have Bird wing, wings whose development varies according to species; the only known groups without wings are the extinct moa and elephant birds. Wings, which are modified forelimbs, gave birds the ability to fly, although further evolution has led to the Flightless bird, loss of flight in some birds, including ratites, penguins, and diverse endemism, endemic island species. The digestive and respiratory systems of birds are also uniquely a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nickel Bis(dimethyldithiocarbamate)
Nickel bis(dimethyldithiocarbamate) is the coordination complex on nickel and dimethyldithiocarbamate, with the formula Ni(S2CNMe2)2 (Me = methyl). It is the prototype for a large number of square planar bis(dialkhyldithiocarbamate)s of nickel(II), which feature diverse organic substituents. Nickel bis(dimethyldithiocarbamate) has been marketed as a fungicide, and related complexes are used as stabilizers in polymers. Preparation and structure The compound precipitates as a light green solid upon combining aqueous solutions of nickel(II) salts and sodium dimethyldithiocarbamate. In terms of structure and bonding, the nickel is square planar, and the complex is diamagnetic. The structure of the closely related nickel bis(diethyldithiocarbamate) has been determined by X-ray crystallography. Oxidation of nickel bis(dieethyldithiocarbamate) gives the red-brown nickel(IV) complex .{{cite journal , last1=Mazumder , first1=Md. Motiur R. , last2=Dalpati , first2=Niharika , last3=Pokk ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron Tris(dimethyldithiocarbamate)
Iron tris(dimethyldithiocarbamate) is the coordination complex of iron with dimethyldithiocarbamate with the formula Fe(S2CNMe2)3 (Me = methyl). It is marketed as a fungicide. Synthesis, structure, bonding Iron tris(dithiocarbamate)s are typically are prepared by salt metathesis reactions. Iron tris(dimethyldithiocarbamate) is an octahedral coordination complex of iron(III) with D3 symmetry. Spin crossover (SCO) was first observed in 1931 by Cambi ''et al.'' who discovered anomalous magnetic behavior for the tris(N,N-dialkyldithiocarbamatoiron(III) complexes. The spin states of these complexes are sensitive to the nature of the amine substituents. Reactions Iron tris(dithiocarbamate)s react with nitric oxide to give a nitrosyl complex: : This efficient chemical trapping reaction provides a means to detect NO. Reflecting the strongly donating properties of dithiocarbamate ligands, iron tris(dithiocarbamate)s oxidize at relatively mild potentials to give isolable iron(IV) deriv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amphibians
Amphibians are ectothermic, anamniote, anamniotic, tetrapod, four-limbed vertebrate animals that constitute the class (biology), class Amphibia. In its broadest sense, it is a paraphyletic group encompassing all Tetrapod, tetrapods, but excluding the amniotes (tetrapods with an amniotic membrane, such as modern reptiles, birds and mammals). All extant taxon, extant (living) amphibians belong to the monophyletic subclass (biology), subclass Lissamphibia, with three living order (biology), orders: Anura (frogs and toads), Urodela (salamanders), and Gymnophiona (caecilians). Evolved to be mostly semiaquatic, amphibians have adapted to inhabit a wide variety of habitats, with most species living in freshwater ecosystem, freshwater, wetland or terrestrial ecosystems (such as riparian woodland, fossorial and even arboreal habitats). Their biological life cycle, life cycle typically starts out as aquatic animal, aquatic larvae with gills known as tadpoles, but some species have devel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pesticide Action Network
Pesticide Action Network (PAN) is an international coalition of more than 600 NGOs in 90 countries which advocates for less hazardous alternatives to pesticides. It was founded in May 1982 with its first meeting in Penang, Malaysia. Origins The origins of PAN have been linked to the start of the "global anti-toxics movement". In 1981 journalist David Weir of The Center for Investigative Reporting, published the book ''The Circle of Poison'' focusing on pesticides, followed a year later by ''A Growing Problem: Pesticides and the Third World Poor'' by David Bull of Oxfam. In 1982, Anwar Fazal, a Malaysian activist who at the time was the first person from a developing country to head the International Organization of Consumers Unions (IOCU; later known as Consumers International), organized a meeting in Penang, Malaysia to explore the possibility of an international network of activists focusing on pesticide regulation. The meeting included Weir and Bull, that represented their ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aquatic Toxicology
Aquatic toxicology is the study of the effects of manufactured chemicals and other anthropogenic and natural materials and activities on aquatic organisms at various levels of organization, from subcellular through individual organisms to communities and ecosystems. Aquatic toxicology is a multidisciplinary field which integrates toxicology, aquatic ecology and aquatic chemistry. This field of study includes freshwater, marine water and sediment environments. Common tests include standardized acute and chronic toxicity tests lasting 24–96 hours (acute test) to 7 days or more (chronic tests). These tests measure endpoints such as survival, growth, reproduction, that are measured at each concentration in a gradient, along with a control test. Typically using selected organisms with ecologically relevant sensitivity to toxicants and a well-established literature background. These organisms can be easily acquired or cultured in lab and are easy to handle. History While basic re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
United States Environmental Protection Agency
The Environmental Protection Agency (EPA) is an independent agency of the United States government tasked with environmental protection matters. President Richard Nixon proposed the establishment of EPA on July 9, 1970; it began operation on December 2, 1970, after Nixon signed an executive order. The order establishing the EPA was ratified by committee hearings in the House and Senate. The agency is led by its administrator, who is appointed by the president and approved by the Senate. The current administrator is Lee Zeldin. The EPA is not a Cabinet department, but the administrator is normally given cabinet rank. The EPA has its headquarters in Washington, D.C. There are regional offices for each of the agency's ten regions, as well as 27 laboratories around the country. The agency conducts environmental assessment, research, and education. It has the responsibility of maintaining and enforcing national standards under a variety of environmental laws, in consultat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs, often through Lewis acids and bases, Lewis bases. The nature of metal–ligand bonding can range from covalent bond, covalent to ionic bond, ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acids and bases, Lewis acidic "ligands". Metals and metalloids are bound to ligands in almost all circumstances, although gaseous "naked" metal ions can be generated in a high vacuum. Ligands in a complex dictate the reactivity (chemistry), reactivity of the central atom, including ligand substitution rates, the reactivity of the ligands themselves, and redox. Ligand selection requires critical consideration in many practical are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
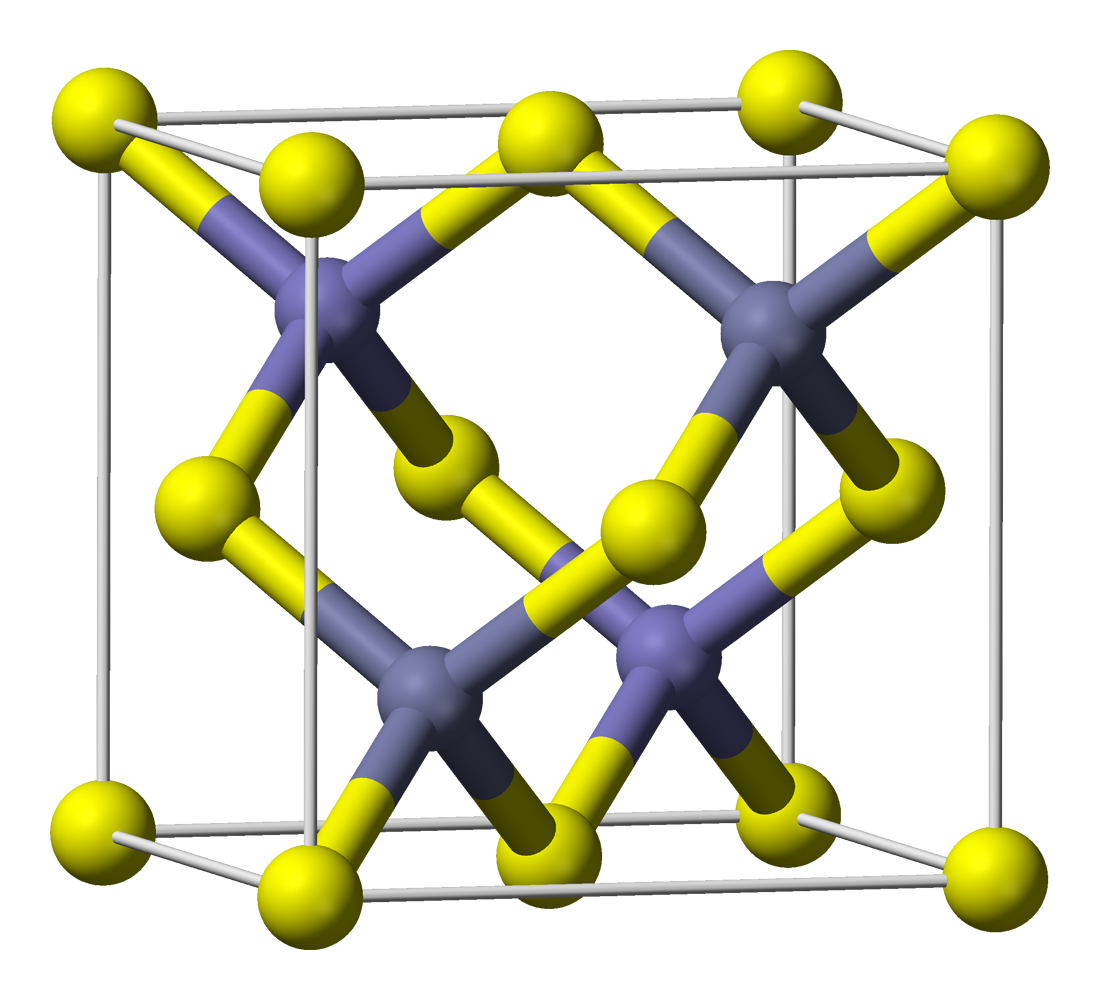
Zinc Sulfide
Zinc sulfide (or zinc sulphide) is an inorganic compound with the chemical formula of ZnS. This is the main form of zinc found in nature, where it mainly occurs as the mineral sphalerite. Although this mineral is usually black because of various impurities, the pure material is white, and it is widely used as a pigment. In its dense synthetic form, zinc sulfide can be transparency and translucency, transparent, and it is used as a window for visible light, visible optics and infrared optics. Structure ZnS exists in two main crystalline forms. This dualism is an example of polymorphism (materials science), polymorphism. In each form, the coordination geometry at Zn and S is tetrahedral. The more stable cubic form is known also as zinc blende or sphalerite. The hexagonal form is known as the mineral wurtzite, although it also can be produced synthetically.. The transition from the sphalerite form to the wurtzite form occurs at around 1020 °C. Applications Luminescent mate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Granular Material
A granular material is a conglomeration of discrete solid, macroscopic scale, macroscopic particles characterized by a loss of energy whenever the particles interact (the most common example would be friction when granulation, grains collide). The constituents that compose granular material are large enough such that they are not subject to thermal motion fluctuations. Thus, the lower size limit for grains in granular material is about 1 micrometre, μm. On the upper size limit, the physics of granular materials may be applied to ice floes where the individual grains are icebergs and to asteroid belts of the Solar System with individual grains being asteroids. Some examples of granular materials are snow, nut (fruit), nuts, coal, sand, rice, coffee, corn flakes, salt, and ball (bearing), bearing balls. Research into granular materials is thus directly applicable and goes back at least to Charles-Augustin de Coulomb, whose Friction, law of friction was originally stated for granul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |