Ethylene oxide is an
organic compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-co ...
with the
formula
In science, a formula is a concise way of expressing information symbolically, as in a mathematical formula or a ''chemical formula''. The informal use of the term ''formula'' in science refers to the general construct of a relationship betwe ...
. It is a cyclic
ether
In organic chemistry, ethers are a class of compounds that contain an ether group, a single oxygen atom bonded to two separate carbon atoms, each part of an organyl group (e.g., alkyl or aryl). They have the general formula , where R and R� ...
and the simplest
epoxide
In organic chemistry, an epoxide is a cyclic ether, where the ether forms a three-atom ring: two atoms of carbon and one atom of oxygen. This triangular structure has substantial ring strain, making epoxides highly reactive, more so than other ...
: a three-membered
ring consisting of one
oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
atom and two
carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
atoms. Ethylene oxide is a colorless and
flammable
A combustible material is a material that can burn (i.e., sustain a flame) in air under certain conditions. A material is flammable if it ignites easily at ambient temperatures. In other words, a combustible material ignites with some effort ...
gas with a faintly sweet odor. Because it is a
strained ring, ethylene oxide easily participates in a number of
addition reaction
In organic chemistry, an addition reaction is an organic reaction in which two or more molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, ...
s that result in ring-opening. Ethylene oxide is
isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formula – that is, the same number of atoms of each element (chemistry), element – but distinct arrangements of atoms in space. ''Isomerism'' refers to the exi ...
ic with
acetaldehyde
Acetaldehyde (IUPAC systematic name ethanal) is an organic compound, organic chemical compound with the chemical formula, formula , sometimes abbreviated as . It is a colorless liquid or gas, boiling near room temperature. It is one of the most ...
and with
vinyl alcohol. Ethylene oxide is industrially produced by
oxidation
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
of
ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon–carbon bond, carbon–carbon doub ...
in the presence of a
silver
Silver is a chemical element; it has Symbol (chemistry), symbol Ag () and atomic number 47. A soft, whitish-gray, lustrous transition metal, it exhibits the highest electrical conductivity, thermal conductivity, and reflectivity of any metal. ...
catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
.
The reactivity that is responsible for many of ethylene oxide's hazards also makes it useful. Although too dangerous for direct household use and generally unfamiliar to consumers, ethylene oxide is used for making many consumer products as well as non-consumer chemicals and intermediates. These products include detergents, thickeners, solvents, plastics, and various organic chemicals such as
ethylene glycol, ethanolamines, simple and complex
glycols,
polyglycol ethers, and other compounds. Although it is a vital raw material with diverse applications, including the manufacture of products like
polysorbate 20 and
polyethylene glycol
Polyethylene glycol (PEG; ) is a polyether compound derived from petroleum with many applications, from industrial manufacturing to medicine. PEG is also known as polyethylene oxide (PEO) or polyoxyethylene (POE), depending on its molecular wei ...
(PEG) that are often more effective and less toxic than alternative materials, ethylene oxide itself is a very hazardous substance. At room temperature it is a very flammable,
carcinogenic
A carcinogen () is any agent that promotes the development of cancer. Carcinogens can include synthetic chemicals, naturally occurring substances, physical agents such as ionizing and non-ionizing radiation, and Biological agent, biologic agent ...
,
mutagenic
In genetics, a mutagen is a physical or chemical agent that permanently changes genetic material, usually DNA, in an organism and thus increases the frequency of mutations above the natural background level. As many mutations can cause cancer in ...
, irritating, and
anaesthetic gas.
[
Ethylene oxide is a surface ]disinfectant
A disinfectant is a chemical substance or compound used to inactivate or destroy microorganisms on inert surfaces. Disinfection does not necessarily kill all microorganisms, especially resistant bacterial spores; it is less effective than ...
that is widely used in hospitals and the medical equipment industry to replace steam in the sterilization of heat-sensitive tools and equipment, such as disposable plastic syringes. It is so flammable and extremely explosive that it is used as a main component of thermobaric weapons;[ therefore, it is commonly handled and shipped as a refrigerated liquid to control its hazardous nature.][Rebsdat, Siegfried and Mayer, Dieter (2005) "Ethylene Oxide" in ''Ullmann's Encyclopedia of Industrial Chemistry''. Wiley-VCH, Weinheim. .]
History
Ethylene oxide was first reported in 1859 by the French chemist Charles-Adolphe Wurtz, who prepared it by treating 2-chloroethanol with potassium hydroxide
Potassium hydroxide is an inorganic compound with the formula K OH, and is commonly called caustic potash.
Along with sodium hydroxide (NaOH), KOH is a prototypical strong base. It has many industrial and niche applications, most of which utili ...
:
:
Wurtz measured the boiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding envi ...
of ethylene oxide as , slightly higher than the present value, and discovered the ability of ethylene oxide to react with acids and salts of metals.electrolyte
An electrolyte is a substance that conducts electricity through the movement of ions, but not through the movement of electrons. This includes most soluble Salt (chemistry), salts, acids, and Base (chemistry), bases, dissolved in a polar solven ...
.ether
In organic chemistry, ethers are a class of compounds that contain an ether group, a single oxygen atom bonded to two separate carbon atoms, each part of an organyl group (e.g., alkyl or aryl). They have the general formula , where R and R� ...
s — particularly by its propensity to engage in the addition reactions typical of unsaturated compounds — had long been a matter of debate. The heterocyclic triangular structure of ethylene oxide was proposed by 1868 or earlier.
Wurtz's 1859 synthesis long remained the only method of preparing ethylene oxide, despite numerous attempts, including by Wurtz himself, to produce ethylene oxide directly from ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon–carbon bond, carbon–carbon doub ...
.silver
Silver is a chemical element; it has Symbol (chemistry), symbol Ag () and atomic number 47. A soft, whitish-gray, lustrous transition metal, it exhibits the highest electrical conductivity, thermal conductivity, and reflectivity of any metal. ...
catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
. Since 1940, almost all industrial production of ethylene oxide has relied on this process. Sterilization by ethylene oxide for the preservation of spice
In the culinary arts, a spice is any seed, fruit, root, Bark (botany), bark, or other plant substance in a form primarily used for flavoring or coloring food. Spices are distinguished from herbs, which are the leaves, flowers, or stems of pl ...
s was patented in 1938 by the American chemist Lloyd Hall. Ethylene oxide achieved industrial importance during World War I
World War I or the First World War (28 July 1914 – 11 November 1918), also known as the Great War, was a World war, global conflict between two coalitions: the Allies of World War I, Allies (or Entente) and the Central Powers. Fighting to ...
as a precursor to both the coolant ethylene glycol and the chemical weapon
A chemical weapon (CW) is a specialized munition that uses chemicals formulated to inflict death or harm on humans. According to the Organisation for the Prohibition of Chemical Weapons (OPCW), this can be any chemical compound intended as ...
mustard gas
Mustard gas or sulfur mustard are names commonly used for the organosulfur compound, organosulfur chemical compound bis(2-chloroethyl) sulfide, which has the chemical structure S(CH2CH2Cl)2, as well as other Chemical species, species. In the wi ...
.
Molecular structure and properties

 The epoxy cycle of ethylene oxide is an almost regular triangle with bond angles of about 60° and a significant angular strain corresponding to the energy of 105 kJ/mol.
The epoxy cycle of ethylene oxide is an almost regular triangle with bond angles of about 60° and a significant angular strain corresponding to the energy of 105 kJ/mol.alcohols
In chemistry, an alcohol (), is a type of organic compound that carries at least one hydroxyl () functional group bound to a Saturated and unsaturated compounds, saturated carbon atom. Alcohols range from the simple, like methanol and ethanol ...
the C–O–H angle is about 110°; in ether
In organic chemistry, ethers are a class of compounds that contain an ether group, a single oxygen atom bonded to two separate carbon atoms, each part of an organyl group (e.g., alkyl or aryl). They have the general formula , where R and R� ...
s, the C–O–C angle is 120°. The moment of inertia
The moment of inertia, otherwise known as the mass moment of inertia, angular/rotational mass, second moment of mass, or most accurately, rotational inertia, of a rigid body is defined relatively to a rotational axis. It is the ratio between ...
about each of the principal axes are ''IA''=, ''IB''= and ''IC''=.
The relative instability of the carbon-oxygen bonds in the molecule is revealed by the comparison in the table of the energy required to break two C–O bonds in the ethylene oxide or one C–O bond in ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the ps ...
and dimethyl ether
Dimethyl ether (DME; also known as methoxymethane) is the organic compound with the formula CH3OCH3,
(sometimes ambiguously simplified to C2H6O as it is an isomer of ethanol). The simplest ether, it is a colorless gas that is a useful precursor ...
:
This instability correlates with its high reactivity, explaining the ease of its ring-opening reaction
A cyclic compound (or ring compound) is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where ...
s (see Chemical properties
A chemical property is any of a material property, material's properties that becomes evident during, or after, a chemical reaction; that is, any attribute that can be established only by changing a substance's chemical substance, chemical identit ...
).
Physical properties
Ethylene oxide is a colorless gas at and is a mobile liquid at – viscosity of liquid ethylene oxide at 0 °C is about 5.5 times lower than that of water. The gas has a characteristic sweet odor of ether, noticeable when its concentration in air exceeds 500ppm.ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the ps ...
, diethyl ether
Diethyl ether, or simply ether, is an organic compound with the chemical formula , sometimes abbreviated as . It is a colourless, highly Volatility (chemistry), volatile, sweet-smelling ("ethereal odour"), extremely flammable liquid. It belongs ...
, and many organic solvents.
Main thermodynamical constants are:surface tension
Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. Surface tension (physics), tension is what allows objects with a higher density than water such as razor blades and insects (e.g. Ge ...
of liquid ethylene oxide, at the interface with its own vapor, is at and at .
* The boiling point increases with the vapor pressure as follows: (), (), and ().
* Viscosity
Viscosity is a measure of a fluid's rate-dependent drag (physics), resistance to a change in shape or to movement of its neighboring portions relative to one another. For liquids, it corresponds to the informal concept of ''thickness''; for e ...
decreases with temperature with the values of 0.577kPa·s at , 0.488 kPa·s at , 0.394kPa·s at , and 0.320kPa·s at .
Between , vapor pressure ''p'' (in mmHg) varies with temperature (''T'' in °C) as
: .
*N/A – data not available.
*N/A – data not available.
Chemical properties
Ethylene oxide readily reacts with diverse compounds with opening of the ring. Its typical reactions are with nucleophiles which proceed via the SN2 mechanism both in acidic (weak nucleophiles: water, alcohols) and alkaline media (strong nucleophiles: OH−, RO−, NH3, RNH2, RR'NH, etc.).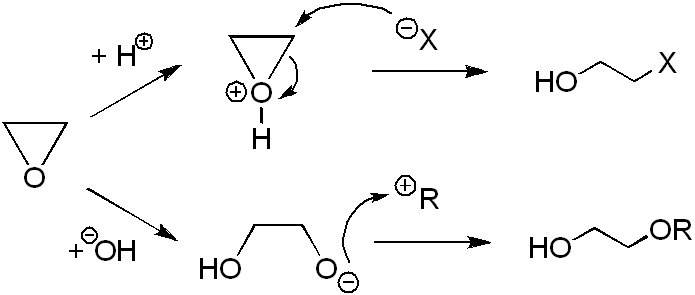 and more specific reactions are described below.
and more specific reactions are described below.
Addition of water and alcohols
Aqueous solutions of ethylene oxide are rather stable and can exist for a long time without any noticeable chemical reaction. However adding a small amount of acid, such as strongly diluted sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
, immediately leads to the formation of ethylene glycol, even at room temperature:
: (CH2CH2)O + H2O → HO–CH2CH2–OH
The reaction also occurs in the gas phase, in the presence of a phosphoric acid
Phosphoric acid (orthophosphoric acid, monophosphoric acid or phosphoric(V) acid) is a colorless, odorless phosphorus-containing solid, and inorganic compound with the chemical formula . It is commonly encountered as an 85% aqueous solution, ...
salt as a catalyst.polyethylene glycol
Polyethylene glycol (PEG; ) is a polyether compound derived from petroleum with many applications, from industrial manufacturing to medicine. PEG is also known as polyethylene oxide (PEO) or polyoxyethylene (POE), depending on its molecular wei ...
:
: n (CH2CH2)O + H2O → HO–(–CH2CH2–O–)n–H
Reactions with alcohols
In chemistry, an alcohol (), is a type of organic compound that carries at least one hydroxyl () functional group bound to a Saturated and unsaturated compounds, saturated carbon atom. Alcohols range from the simple, like methanol and ethanol ...
proceed similarly yielding ethylene glycol ethers:
: (CH2CH2)O + C2H5OH → HO–CH2CH2–OC2H5
: 2 (CH2CH2)O + C2H5OH → HO–CH2CH2–O–CH2CH2–OC2H5
Reactions with lower alcohols occur less actively than with water and require more severe conditions, such as heating to and pressurizing to and adding an acid or alkali catalyst.
Reactions of ethylene oxide with fatty alcohols proceed in the presence of sodium
Sodium is a chemical element; it has Symbol (chemistry), symbol Na (from Neo-Latin ) and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 element, group 1 of the peri ...
metal, sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula . It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly corrosive base (chemistry), ...
, or boron trifluoride and are used for the synthesis of surfactants
Surfactants are chemical compounds that decrease the surface tension or interfacial tension between two liquids, a liquid and a gas, or a liquid and a solid. The word ''surfactant'' is a blend of "surface-active agent",
coined in 1950. As t ...
.
Addition of carboxylic acids and their derivatives
Reactions of ethylene oxide with carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an Substituent, R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl ...
s in the presence of a catalyst results in glycol mono- and diesters:
: (CH2CH2)O + CH3CO2H → HOCH2CH2–O2CCH3
: (CH2CH2)O + (CH3CO)2O → CH3CO2CH2CH2O2CCH3
The addition of acid amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a chemical compound, compound with the general formula , where R, R', and R″ represent any group, typically organyl functional group, groups or hydrogen at ...
s proceeds similarly:
: (CH2CH2)O + CH3CONH2 → HOCH2CH2NHC(O)CH3
Addition of ethylene oxide to higher carboxylic acids is carried out at elevated temperatures (typically ) and pressure () in an inert atmosphere, in presence of an alkaline catalyst (concentration 0.01–2%), such as hydroxide or carbonate of sodium or potassium. The carboxylate ion acts as nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
in the reaction:
: (CH2CH2)O + RCO2− → RCO2CH2CH2O−
: RCO2CH2CH2O− + RCO2H → RCO2CH2CH2OH + RCO2−
Adding ammonia and amines
Ethylene oxide reacts with ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
forming a mixture of mono-, di-, and tri- ethanolamines. The reaction is stimulated by adding a small amount of water.
: (CH2CH2)O + NH3 → HO–CH2CH2–NH2
: 2 (CH2CH2)O + NH3 → (HO–CH2CH2)2NH
: 3 (CH2CH2)O + NH3 → (HO–CH2CH2)3N
Similarly proceed the reactions with primary and secondary amines:
: (CH2CH2)O + RNH2 → HO–CH2CH2–NHR
Dialkylamino ethanols can further react with ethylene oxide, forming amino polyethylene glycols:Trimethylamine
Trimethylamine (TMA) is an organic compound with the formula N(CH3)3. It is a trimethylated derivative of ammonia. TMA is widely used in industry. At higher concentrations it has an ammonia-like odor, and can cause necrosis of mucous membranes ...
reacts with ethylene oxide in the presence of water, forming choline:
: (CH2CH2)O + (CH3)3N + H2O → OCH2CH2N (CH3)3sup>+OH−
Aromatic primary and secondary amines also react with ethylene oxide, forming the corresponding arylamino alcohols.
Halide addition
Ethylene oxide readily reacts with aqueous solutions of hydrochloric, hydrobromic, and hydroiodic acids to form halohydrins. The reaction occurs easier with the last two acids:
: (CH2CH2)O + HCl → HO–CH2CH2–Cl
The reaction with these acids competes with the acid-catalyzed hydration of ethylene oxide; therefore, there is always a by-product of ethylene glycol with an admixture of diethylene glycol. For a cleaner product, the reaction is conducted in the gas phase or in an organic solvent.
Ethylene fluorohydrin is obtained differently, by boiling hydrogen fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluori ...
with a 5–6% solution of ethylene oxide in diethyl ether
Diethyl ether, or simply ether, is an organic compound with the chemical formula , sometimes abbreviated as . It is a colourless, highly Volatility (chemistry), volatile, sweet-smelling ("ethereal odour"), extremely flammable liquid. It belongs ...
. The ether normally has a water content of 1.5–2%; in absence of water, ethylene oxide polymerizes.
Halohydrins can also be obtained by passing ethylene oxide through aqueous solutions of metal halides:
Metalorganic addition
Interaction of ethylene oxide with organomagnesium compounds, which are Grignard reagents, can be regarded as nucleophilic substitution
In chemistry, a nucleophilic substitution (SN) is a class of chemical reactions in which an electron-rich chemical species (known as a nucleophile) replaces a functional group within another electron-deficient molecule (known as the electrophile) ...
influenced by carbanion organometallic compounds. The final product of the reaction is a primary alcohol:
: (CH2CH2)O + RMgBr -> R-CH2CH2-OMgBr -> ce\overset
Similar mechanism is valid for other organometallic compounds, such as alkyl lithium:
: (CH2CH2)O + \overset -> R-CH2CH2-OLi -> ceR-CH2CH2-OH
Other addition reactions
Addition of hydrogen cyanide
Ethylene oxide easily reacts with hydrogen cyanide
Hydrogen cyanide (formerly known as prussic acid) is a chemical compound with the chemical formula, formula HCN and structural formula . It is a highly toxic and flammable liquid that boiling, boils slightly above room temperature, at . HCN is ...
forming ethylene cyanohydrin:
: (CH2CH2)O + HCN → HO–CH2CH2–CN
A slightly chilled (10–20 °C) aqueous solution of calcium cyanide can be used instead of HCN:
: 2 (CH2CH2)O + Ca(CN)2 + 2 H2O → 2 HO–CH2CH2–CN + Ca(OH)2
Ethylene cyanohydrin easily loses water, producing acrylonitrile:
: HO–CH2CH2–CN → CH2=CH–CN + H2O
Addition of hydrogen sulfide and mercaptans
When reacting with the hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is toxic, corrosive, and flammable. Trace amounts in ambient atmosphere have a characteristic foul odor of rotten eggs. Swedish chemist ...
, ethylene oxide forms 2-mercaptoethanol and thiodiglycol, and with alkylmercaptans it produces 2-alkyl mercaptoetanol:
: (CH2CH2)O + H2S → HO–CH2CH2–HS
: 2 (CH2CH2)O + H2S → (HO–CH2CH2)2S
: (CH2CH2)O + RHS → HO–CH2CH2–SR
The excess of ethylene oxide with an aqueous solution of hydrogen sulfide leads to the tris-(hydroxyethyl) sulfonyl hydroxide:
: 3 (CH2CH2)O + H2S → HO–CH2CH2)3S+H−
Addition of nitrous and nitric acids
Reaction of ethylene oxide with aqueous solutions of barium nitrite, calcium nitrite, magnesium nitrite, zinc nitrite, or sodium nitrite leads to the formation of 2-nitroethanol:
:2 (CH2CH2)O + Ca(NO2)2 + 2 H2O → 2 HO–CH2CH2–NO2 + Ca(OH)2
With nitric acid
Nitric acid is an inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into nitrogen oxide, oxides of nitrogen. Most com ...
, ethylene oxide forms mono- and dinitroglycols:
: (CH2CH2)O + \overset -> HO-CH2CH2-ONO2 -> ce ceO2NO-CH2CH2-ONO_2
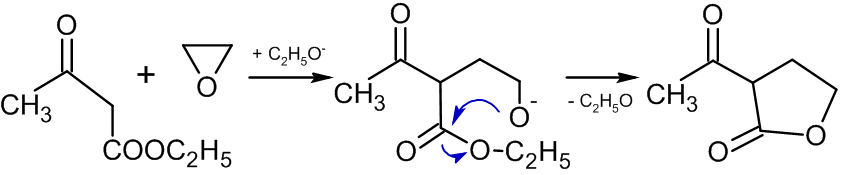
Reaction with compounds containing active methylene groups
In the presence of alkoxide
In chemistry, an alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They are written as , where R is the organyl substituent. Alkoxides are strong bases and, whe ...
s, reactions of ethylene oxide with compounds containing active methylene group leads to the formation of butyrolactones:
: 
Alkylation of aromatic compounds
Ethylene oxide enters into the Friedel–Crafts reaction
The Friedel–Crafts reactions are a set of organic reaction, reactions developed by Charles Friedel and James Crafts in 1877 to attach substituents to an Aromatic hydrocarbon, aromatic ring. Friedel–Crafts reactions are of two main types: alky ...
with benzene to form phenethyl alcohol:
:
Styrene
Styrene is an organic compound with the chemical formula C6H5CH=CH2. Its structure consists of a vinyl group as substituent on benzene. Styrene is a colorless, oily liquid, although aged samples can appear yellowish. The compound evaporates easi ...
can be obtained in one stage if this reaction is conducted at elevated temperatures () and pressures (), in presence of an aluminosilicate catalyst.
Synthesis of crown ethers
A series of polynomial heterocyclic compounds, known as crown ethers, can be synthesized with ethylene oxide. One method is the cationic cyclopolymerization of ethylene oxide, limiting the size of the formed cycle:sulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a colorless gas with a pungent smell that is responsible for the odor of burnt matches. It is r ...
in the presence of caesium salts leads to the formation of an 11-membered heterocyclic compound which has the complexing properties of crown ethers: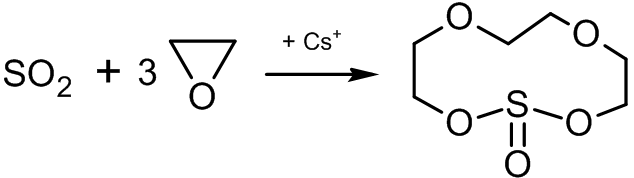
Isomerization
When heated to about , or to in the presence of a catalyst ( Al2O3, H3PO4, etc.), ethylene oxide isomerizes into acetaldehyde
Acetaldehyde (IUPAC systematic name ethanal) is an organic compound, organic chemical compound with the chemical formula, formula , sometimes abbreviated as . It is a colorless liquid or gas, boiling near room temperature. It is one of the most ...
:binding energy
In physics and chemistry, binding energy is the smallest amount of energy required to remove a particle from a system of particles or to disassemble a system of particles into individual parts. In the former meaning the term is predominantly use ...
of the C-C bond in acetaldehyde.
Reduction reaction
Ethylene oxide can be hydrogenated into ethanol in the presence of a catalyst, such as nickel
Nickel is a chemical element; it has symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive, but large pieces are slo ...
, platinum
Platinum is a chemical element; it has Symbol (chemistry), symbol Pt and atomic number 78. It is a density, dense, malleable, ductility, ductile, highly unreactive, precious metal, precious, silverish-white transition metal. Its name origina ...
, palladium
Palladium is a chemical element; it has symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1802 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas (formally 2 Pallas), ...
,lithium aluminium hydride
Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula or . It is a white solid, discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthe ...
, and some other hydride
In chemistry, a hydride is formally the anion of hydrogen (H−), a hydrogen ion with two electrons. In modern usage, this is typically only used for ionic bonds, but it is sometimes (and has been more frequently in the past) applied to all che ...
s.acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main compone ...
, of lithium aluminium hydride with titanium trichloride (the reducing agent is actually titanium dichloride, formed by the reaction between LiAlH4 and TiCl3) and of iron(III) chloride with butyllithium Butyllithium may refer to one of 5 isomeric organolithium reagents of which 3 are commonly used in chemical synthesis:
* ''n''-Butyllithium, abbreviated BuLi or nBuLi
* ''sec''-Butyllithium, abbreviated ''sec''-BuLi or sBuLi, has 2 stereoisomers, ...
in tetrahydrofuran
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water- miscible organic liquid with low viscosity. It is ...
.
Oxidation
Ethylene oxide can further be oxidized, depending on the conditions, to glycolic acid
Glycolic acid (or hydroxyacetic acid; chemical formula ) is a colorless, odorless and hygroscopic crystal, crystalline solid, highly solubility, soluble in water. It is used in various skin care, skin-care products. Glycolic acid is widespread in ...
or carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
:
: (CH2CH2)O + O2 -> ce\overset
Deep gas-phase reactor oxidation of ethylene oxide at and a pressure of yields a complex mixture of products containing O2, H2, CO, CO2, CH4, C2H2, C2H4, C2H6, C3H6, C3H8, and CH3CHO.
Dimerization
In the presence of acid catalysts, ethylene oxide dimerizes to afford dioxane:
: 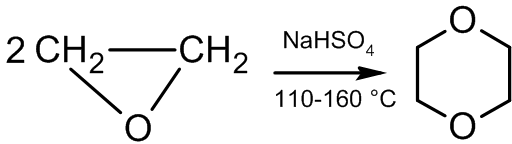 The reaction mechanism is as follows:
The reaction mechanism is as follows: The dimerization reaction is unselective. By-products include
The dimerization reaction is unselective. By-products include acetaldehyde
Acetaldehyde (IUPAC systematic name ethanal) is an organic compound, organic chemical compound with the chemical formula, formula , sometimes abbreviated as . It is a colorless liquid or gas, boiling near room temperature. It is one of the most ...
(due to isomerization
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomer ...
). The selectivity and speed of dimerization can be increased by adding a catalyst, such as platinum, platinum-palladium, or iodine
Iodine is a chemical element; it has symbol I and atomic number 53. The heaviest of the stable halogens, it exists at standard conditions as a semi-lustrous, non-metallic solid that melts to form a deep violet liquid at , and boils to a vi ...
with sulfolane
Sulfolane (also tetramethylene sulfone, IUPAC nomenclature, systematic name: 1λ6-thiolane-1,1-dione) is an organosulfur compound, formally a cyclic sulfone, with the formula . It is a colorless liquid commonly used in the chemical industry as a s ...
. 2-methyl-1,3-dioxolane is formed as a side product in the last case.
Polymerization
Liquid ethylene oxide can form polyethylene glycol
Polyethylene glycol (PEG; ) is a polyether compound derived from petroleum with many applications, from industrial manufacturing to medicine. PEG is also known as polyethylene oxide (PEO) or polyoxyethylene (POE), depending on its molecular wei ...
s. The polymerization can proceed via radical and ionic mechanisms, but only the latter has a wide practical application.alkoxide
In chemistry, an alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They are written as , where R is the organyl substituent. Alkoxides are strong bases and, whe ...
s, hydroxides, carbonates, or other compounds of alkali or alkaline earth metals.
Thermal decomposition
Ethylene oxide is relatively stable to heating – in the absence of a catalyst, it does not dissociate up to , and only above there is a major exothermic decomposition, which proceeds through the radical mechanism.isomerization
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomer ...
, however high temperature accelerates the radical processes. They result in a gas mixture containing acetaldehyde, ethane, ethyl, methane, hydrogen, carbon dioxide, ketene, and formaldehyde. High-temperature pyrolysis () at elevated pressure in an inert atmosphere leads to a more complex composition of the gas mixture, which also contains acetylene and propane.
Other reactions
Thiocyanate ions or thiourea transform ethylene oxide into thiirane (ethylene sulfide):
: (CH2CH2)O + (NH2)2C=S → (CH2CH2)S + (NH2)2C=O
:  Reaction of phosphorus pentachloride with ethylene oxide produces ethylene dichloride:
Reaction of phosphorus pentachloride with ethylene oxide produces ethylene dichloride:[
]
Phosphorus trichloride reacts with ethylene oxide forming chloroethyl esters of phosphorous acid:carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
, in a non-polar solvent in the presence of ''bis''-(triphenylphosphine)-nickel(0) results in ethylene carbonate:
:  In industry, a similar reaction is carried out at high pressure and temperature in the presence of quaternary ammonium or phosphonium salts as a catalyst.
Reaction of ethylene oxide with formaldehyde at 80–150 °C in the presence of a catalyst leads to the formation of dioxolane, 1,3-dioxolane:
In industry, a similar reaction is carried out at high pressure and temperature in the presence of quaternary ammonium or phosphonium salts as a catalyst.
Reaction of ethylene oxide with formaldehyde at 80–150 °C in the presence of a catalyst leads to the formation of dioxolane, 1,3-dioxolane: Substituting formaldehyde by other aldehydes or ketones results in a 2-substituted 1,3-dioxolane (yield: 70–85%, catalyst: tetraethylammonium bromide).
Substituting formaldehyde by other aldehydes or ketones results in a 2-substituted 1,3-dioxolane (yield: 70–85%, catalyst: tetraethylammonium bromide).
Laboratory synthesis
Dehydrochlorination of ethylene and its derivatives
Dehydrochlorination of 2-chloroethanol, developed by Wurtz in 1859, remains a common laboratory route to ethylene oxide:
: Cl-CH2CH2-OH + NaOH -> (CH2CH2)O + NaCl + H2O
The reaction is carried out at elevated temperature, and beside sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula . It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly corrosive base (chemistry), ...
or potassium hydroxide
Potassium hydroxide is an inorganic compound with the formula K OH, and is commonly called caustic potash.
Along with sodium hydroxide (NaOH), KOH is a prototypical strong base. It has many industrial and niche applications, most of which utili ...
, calcium hydroxide, barium hydroxide, magnesium hydroxide, or carbonates of alkali or alkaline earth metals can be used.
Direct oxidation of ethylene by peroxy acids
Ethylene can be directly oxidized into ethylene oxide using peroxy acids, for example, peroxybenzoic acid, peroxybenzoic or ''meta''-chloro-peroxybenzoic acid:
:  Oxidation by peroxy acids is efficient for higher alkenes, but not for ethylene. The above reaction is slow and has low yield, therefore it is not used in the industry.
Oxidation by peroxy acids is efficient for higher alkenes, but not for ethylene. The above reaction is slow and has low yield, therefore it is not used in the industry.
Other preparative methods
Other synthesis methods include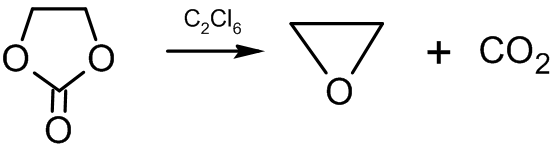
Industrial synthesis
History
Commercial production of ethylene oxide dates back to 1914 when BASF built the first factory which used the chlorohydrin process (reaction of ethylene chlorohydrin with calcium hydroxide). The chlorohydrin process was unattractive for several reasons, including low efficiency and loss of valuable chlorine into calcium chloride. More efficient direct oxidation of ethylene by air was invented by Lefort in 1931 and in 1937 Union Carbide opened the first plant using this process. It was further improved in 1958 by Shell Oil Co. by replacing air with oxygen and using elevated temperature of and pressure ().
Chlorohydrin process of production of ethylene oxide
Although the chlorohydrin process is almost entirely superseded in the industry by the direct oxidation of ethylene, the knowledge of this method is still important for educational reasons and because it is still used in the production of propylene oxide. The process consists of three major steps: synthesis of ethylene chlorohydrin, dehydrochlorination of ethylene chlorohydrin to ethylene oxide and purification of ethylene oxide. Those steps are carried continuously. In the first column, hypochlorination of ethylene is carried out as follows:
Direct oxidation of ethylene
Usage in global industry
Direct oxidation of ethylene was patented by Lefort in 1931. This method was repeatedly modified for industrial use, and at least four major variations are known. They all use oxidation by oxygen or air and a silver-based catalyst, but differ in the technological details and hardware implementations.
Chemistry and kinetics of the direct oxidation process
Formally, the direct oxidation process is expressed by the following equation:
: 2CH_2=CH2 + O2 -> ce2(CH2CH2)O, ΔH=−105 kJ/mol
However, significant yield of carbon dioxide and water is observed in practice, which can be explained by the complete oxidation of ethylene or ethylene oxide:
: CH2=CH2 + 3 O2 → 2 CO2 + 2 H2O, ΔH=−1327kJ/mol
: (CH2CH2)O + 2.5 O2 → 2 CO2 + 2 H2O, ΔH=−1223kJ/mol
According to a kinetic analysis by Kilty and Sachtler, the following reactions describe the pathway leading to EO. In the first step, a superoxide (O2−) species is formed:
Process overview
The production of ethylene oxide on a commercial scale is attained with the unification of the following unit operations, unit processes:
* Main reactor
* Ethylene oxide scrubber
* Ethylene oxide de-sorber
* Stripping (chemistry), Stripping and distillation column
* CO2 scrubber and CO2 de-scrubber
Main Reactor: The main reactor consists of thousands of catalyst tubes in bundles. These tubes are generally long with an inner diameter of . The catalyst packed in these tubes is in the form of spheres or rings of diameter . The operating conditions of with a pressure of prevail in the reactor. To maintain this temperature, the cooling system of the reactor plays a vital role. With the aging of the catalyst, its selectivity decreases and it produces more exothermic side products of CO2.
Ethylene oxide scrubber: After the gaseous stream from the main reactor, containing ethylene oxide (1–2%) and CO2 (5%), is cooled, it is then passed to the ethylene oxide scrubber. Here, water is used as the scrubbing media which scrubs away majority of ethylene oxide along with some amounts of CO2, N2, CH2=CH2, CH4 and aldehydes (introduced by the recycle stream). Also, a small proportion of the gas leaving the ethylene oxide scrubber (0.1–0.2%) is removed continuously (combusted) to prevent the buildup of inert compounds (N2, Ar, and C2H6), which are introduced as impurities with the reactants.
Ethylene oxide de-sorber: The aqueous stream resulting from the above scrubbing process is then sent to the ethylene oxide de-sorber. Here, ethylene oxide is obtained as the overhead product, whereas the bottom product obtained is known as the ''glycol bleed''. When ethylene oxide is scrubbed from the recycle gas with an aqueous solution, ethylene glycols (viz. mono-ethylene glycol, di-ethylene glycol and other poly-ethylene glycols) get unavoidably produced. Thus, in-order to prevent them from building up in the system, they are continuously bled off.
Stripping and distillation column: Here, the ethylene oxide stream is stripped off its low boiling components and then distilled in-order to separate it into water and ethylene oxide.
CO2 scrubber: The recycle stream obtained from the ethylene oxide scrubber is compressed and a side-stream is fed to the CO2 scrubber. Here, CO2 gets dissolved into the hot aqueous solution of potassium carbonate (i.e., the scrubbing media). The dissolution of CO2 is not only a physical phenomenon, but a chemical phenomenon as well, for, the CO2 reacts with potassium carbonate to produce potassium hydrogen carbonate.
: K2CO3 + CO2 + H2O → 2 KHCO3
CO2 de-scrubber: The above potassium carbonate solution (enriched with CO2) is then sent to the CO2 de-scrubber where CO2 is de-scrubbed by stepwise (usually two steps) flash evaporation, flashing. The first step is done to remove the hydrocarbon gases, and the second step is employed to strip off CO2.
World production of ethylene oxide
The world production of ethylene oxide was in 2009,[ in 2008 and in 2007.]
Applications
 Ethylene oxide is one of the most important raw materials used in large-scale chemical production. Most ethylene oxide is used for synthesis of ethylene glycols, including diethylene glycol and triethylene glycol, that accounts for up to 75% of global consumption. Other important products include ethylene glycol ethers, ethanolamines, and ethoxylates. Among glycols, ethylene glycol is used as antifreeze, in the production of polyester and polyethylene terephthalate (PET – raw material for plastic bottles), liquid coolants, and solvents.
Polyethyleneglycols are used in perfumes, cosmetics, pharmaceuticals, lubricants, paint thinners, and plasticizers. Ethylene glycol ethers are part of brake fluids, detergents, solvents, lacquers, and paints. Ethanolamines are used in the manufacture of soap and detergents and for purification of natural gas. Ethoxylates are reaction products of ethylene oxide with higher alcohols, acids, or amines. They are used in the manufacture of detergents, surfactants, emulsifiers, and dispersants.
Whereas synthesis of ethylene glycols is the major application of ethylene oxide, its percentage varies greatly depending on the region: from 44% in the Western Europe, 63% in Japan, and 73% in North America to 90% in the rest of Asia, and 99% in Africa.
Ethylene oxide is one of the most important raw materials used in large-scale chemical production. Most ethylene oxide is used for synthesis of ethylene glycols, including diethylene glycol and triethylene glycol, that accounts for up to 75% of global consumption. Other important products include ethylene glycol ethers, ethanolamines, and ethoxylates. Among glycols, ethylene glycol is used as antifreeze, in the production of polyester and polyethylene terephthalate (PET – raw material for plastic bottles), liquid coolants, and solvents.
Polyethyleneglycols are used in perfumes, cosmetics, pharmaceuticals, lubricants, paint thinners, and plasticizers. Ethylene glycol ethers are part of brake fluids, detergents, solvents, lacquers, and paints. Ethanolamines are used in the manufacture of soap and detergents and for purification of natural gas. Ethoxylates are reaction products of ethylene oxide with higher alcohols, acids, or amines. They are used in the manufacture of detergents, surfactants, emulsifiers, and dispersants.
Whereas synthesis of ethylene glycols is the major application of ethylene oxide, its percentage varies greatly depending on the region: from 44% in the Western Europe, 63% in Japan, and 73% in North America to 90% in the rest of Asia, and 99% in Africa.
Production of ethylene glycol
Ethylene glycol is industrially produced by non-catalytic hydration of ethylene oxide at a temperature of and a pressure of :
Production of glycol ethers
The major industrial esters of mono-, di-, and triethylene glycols are methyl, ethyl, and normal butyl ethers, as well as their acetates and phthalates. The synthesis involves reaction of the appropriate alcohol (chemistry), alcohol with ethylene oxide:
: (CH2CH2)O + ROH -> HOCH2CH2OR
: (CH2CH2)O + HOCH2CH2OR -> HOCH2CH2OCH2CH2OR
: (CH2CH2)O + HOCH2CH2OCH2CH2OR -> HOCH2CH2OCH2CH2OCH2CH2OR
The reaction of monoesters with an acid or its anhydride leads to the formation of the esters:
: CH3CO2H + HOCH2CH2OR -> ROCH2CH2OCOCH3 + H2O
Production of ethanolamines
In the industry, ethanolamines (mono-, di-, and triethanolamines) are produced by reacting ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
and ethylene oxide in anhydrous medium at a temperature of and pressure of MPa:
: (CH2CH2)O + NH3 -> HOCH2CH2NH2
: 2 (CH2CH2)O + NH3 -> (HOCH2CH2)2NH
: 3 (CH2CH2)O + NH3 -> (HOCH2CH2)3N
All three ethanolamines are produced in the process, while ammonia and part of methylamine are recycled. The final products are separated by vacuum distillation. Hydroxyalkylamines are produced in a similar process:
: (CH2CH2)O + RNH2 -> HOCH2CH2NHR
: 2 (CH2CH2)O + RNH2 -> (HOCH2CH2)2NR
Monosubstituted products are formed by reacting a large excess of amine with ethylene oxide in presence of water and at a temperature below . Disubstituted products are obtained with a small excess of ethylene oxide, at a temperature of and a pressure of .
Production of ethoxylates
Industrial production of ethoxylates is realized by a direct reaction of higher alcohols, acids, or amines with ethylene oxide in the presence of an alkaline catalyst at a temperature of . Modern plants producing ethoxylates are usually based on the BUSS LOOP reactors technology,
Production of acrylonitrile
Currently, most acrylonitrile (90% in 2008) is produced by the SOHIO method, which is based on the catalytic oxidation of propylene in the presence of ammonia and bismuth phosphomolybdate. However, until 1960 a key production process was addition of hydrogen cyanide
Hydrogen cyanide (formerly known as prussic acid) is a chemical compound with the chemical formula, formula HCN and structural formula . It is a highly toxic and flammable liquid that boiling, boils slightly above room temperature, at . HCN is ...
to ethylene oxide, followed by dehydration of the resulting cyanohydrin:sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula . It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly corrosive base (chemistry), ...
and diethylamine), and dehydration of cyanohydrin occurs in the gas phase upon the catalytic action of aluminium oxide.
Non-industrial uses
The direct use of ethylene oxide accounts for only 0.05% (2004 data) of its global production.
Healthcare sterilant
 Ethylene oxide is one of the most commonly used sterilization methods in the healthcare industry because of its non-damaging effects for delicate instruments and devices that require sterilization, and for its wide range of material compatibility. It is used for instruments that cannot tolerate heat, moisture, or abrasive chemicals, such as electronics, optical equipment, paper, rubber, and plastics. It was developed in the 1940s as a sterilant by the US military, and its use as a medical sterilant dates to the late 1950s, when the McDonald process was patented for medical devices. The Anprolene system was patented in the 1960s by Andersen Products, and it remains the most commonly used system in several niche markets, notably the veterinary market and some international markets. It relies on the use of a flexible sterilization chamber and an EtO cartridge for small volume sterilization, and where environmental and/or portability considerations dictate the use of a low dose. It is therefore referred to as the "flexible chamber sterilization" method, or the "gas diffusion sterilization" method.
In the United States, the operation of EtO sterilization is overseen by the EPA through the National Emissions Standards for Hazardous Air Pollutants (NESHAP).
Ethylene oxide is one of the most commonly used sterilization methods in the healthcare industry because of its non-damaging effects for delicate instruments and devices that require sterilization, and for its wide range of material compatibility. It is used for instruments that cannot tolerate heat, moisture, or abrasive chemicals, such as electronics, optical equipment, paper, rubber, and plastics. It was developed in the 1940s as a sterilant by the US military, and its use as a medical sterilant dates to the late 1950s, when the McDonald process was patented for medical devices. The Anprolene system was patented in the 1960s by Andersen Products, and it remains the most commonly used system in several niche markets, notably the veterinary market and some international markets. It relies on the use of a flexible sterilization chamber and an EtO cartridge for small volume sterilization, and where environmental and/or portability considerations dictate the use of a low dose. It is therefore referred to as the "flexible chamber sterilization" method, or the "gas diffusion sterilization" method.
In the United States, the operation of EtO sterilization is overseen by the EPA through the National Emissions Standards for Hazardous Air Pollutants (NESHAP).
Niche uses
Ethylene oxide is used as a fungicide and as an accelerator of maturation of tobacco leaves.
Identification of ethylene oxide
Gas chromatography is the principal method for analysis and detection of ethylene oxide.
Accidents
Ethylene oxide is extremely flammable, and its mixtures with air are explosive. When heated it may rapidly expand, causing fire and explosion. Several industrial accidents have been attributed to ethylene oxide explosion.
The autoignition temperature is , thermal decomposition, decomposition temperature of at , minimum inflammable content in the air is 2.7%, and maximum limit is 100%. The NFPA 704 rating is Health, 3; Flammability, 4; Instability 2. Ethylene oxide in presence of water can hydrolyze to ethylene glycol and form polyethylene oxide, which then eventually is oxidized by air and leads to hot spot effect in subatomic physics, hotspots that can trigger explosive decomposition.
Fires caused by ethylene oxide are extinguished with conventional media including fire retardant foam, foam, carbon dioxide, or water. Suppression of this activity can be done by blanketing with an inert gas until total pressure reaches the nonexplosive range. Extinguishing of burning ethylene oxide is complicated by its ability to continue burning in an inert atmosphere and in water solutions. Fire suppression is reached only upon dilution with water above 22:1.
La Canonja, Spain accident
On 14 January 2020 in an industrial estate near Tarragona, an explosion of an ethoxylation reactor owned by the chemical company Industrias Quimicas de Oxido de Etileno (IQOXE, part of the CL Industrial Group) occurred. The accident launched substantial debris over a radius of about two and a half kilometers, one piece penetrating a distant home and killing an occupant. It is reported that at least three people were killed and seven injured as a direct result of the explosion.
The company was, until the time of the explosion the only producer of ethylene oxide in Spain with an installed capacity of 140,000 tons/year. Half of that production was used to manufacture ethylene glycol for PET production. The accident will be investigated under EU regulations within the context of the European Agency for Safety and Health at Work.
2020 sesame seeds contamination
In September 2020, high levels of pesticides were found in 268 tonnes of sesame seeds from India. The contamination had a level of 1000 to 3500 times the limit of 0.05 milligrams per kilogram for ethylene oxide allowed in Europe. This pesticide is forbidden in Europe, where it is recognized to be carcinogenic
A carcinogen () is any agent that promotes the development of cancer. Carcinogens can include synthetic chemicals, naturally occurring substances, physical agents such as ionizing and non-ionizing radiation, and Biological agent, biologic agent ...
and mutagenic. A product recall was made, half of the products had an organic certification.
In September, alert was raised by Belgium by RASFF, but the product has also been sold in other EU single market countries such as France and Ireland.
Long term exposure of residents living near medical supply warehouses.
Residents living near Cardinal Health's warehouse which holds sterilized medical supplies in El Paso Texas reported respiratory symptoms 2021. Some sources estimate these large warehouses holding sterilized medical equipment out gasses more ethylene oxide than some sterilization plant due to laxer regulations. Further investigation discovered people living near these warehouses are being exposed to ethylene oxide levels greater than 1 in 10,000, the EPA's acceptability threshold.
Physiological effects
Effect on microorganisms
Exposure to ethylene oxide gas causes alkylation to microorganisms at a nuclear level. The disinfectant effect of ethylene oxide is similar to that of sterilization by heat, but because of limited penetration, it affects only the surface. ETO sterilization can take up to 12 hours due to its slow action upon microorganisms, and lengthy processing and aeration time.
Effects on humans and animals
Ethylene oxide is an alkylation, alkylating agent; it has irritating, sensitizing, and narcotic effects.[Toxicological Profile For Ethylene Oxide]
Agency for Toxic Substances and Disease Registry, US Public Health Services An increased incidence of peritoneal mesotheliomas was also observed in the animals exposed to concentrations of . Results of human epidemiological studies on workers exposed to ethylene oxide differ. There is evidence from both human and animal studies that inhalation exposure to ethylene oxide can result in a wide range of carcinogenic effects.
Ethylene oxide is toxic by inhalation, with a US Occupational Safety and Health Administration, OSHA permissible exposure limit calculated as a TWA (time weighted average) over 8 hours of 1ppm, and a short term exposure limit (excursion limit) calculated as a TWA over 15 minutes of 5ppm.diethyl ether
Diethyl ether, or simply ether, is an organic compound with the chemical formula , sometimes abbreviated as . It is a colourless, highly Volatility (chemistry), volatile, sweet-smelling ("ethereal odour"), extremely flammable liquid. It belongs ...
, a common laboratory solvent of very low toxicity. In view of these insidious properties, continuous electrochemical monitoring is standard practice, and it is forbidden to use ethylene oxide to fumigate building interiors in the European Union, EU and some other jurisdictions.[ The metabolism of ethylene oxide is not completely known. Data from animal studies indicate two possible pathways for the metabolism of ethylene oxide: hydrolysis to ethylene glycol and glutathione conjugation to form mercapturic acid and meththio-metabolites.
Ethylene oxide easily penetrates through ordinary clothing and footwear, causing skin irritation and dermatitis with the formation of blisters, fever, and leukocytosis.][Codes]
.
* Eye exposure: /6 hours (rabbit)
* Oral: (rat, median lethal dose, LD50), (rat, Lowest published toxic dose, TDLo), (rat, Toxic dose, TD)
* Inhalation: 12,500 ppm (human, Median lethal dose#Other measures of toxicity, TCLo), 960 ppm/4 hours (dog, median lethal dose, LC50) 33–50 ppm (rat or mouse, TC), 800 ppm/4 hours (rat or mouse, LC50)
* Subcutaneous injection: (cat, LDLo), (mouse, TDLo) (mouse, TD), (rat, LD50).
* Intraperitoneal injection: (mouse, TDLo), (mouse, LD50)
* Intravenous injection: (rabbit, LD50), (mouse, LD50)
* The US Environmental Protection Agency (USEPA) estimated in 2016 that for low doses, the inhalation of ethylene oxide for a lifetime could increase an individual's lifetime cancer risk by as much as 3.0×10−3 per μg/m3 (without considering that early-life exposures are likely more potent). The USEPA estimated the slope of the dose-response declines at higher doses, and extra cancer risk estimates for several occupational exposure scenarios are calculated.
Global demand
Global EO demand has expanded from in 2004 to in 2009, while demand for refined EO expanded from in 2004 to in 2008. In 2009, demand is estimated to have declined to about . Total EO demand registered a growth rate of 5.6% per annum during the period 2005 to 2009 and is projected to grow at 5.7% per annum during 2009 to 2013.
Health and safety regulations
According to Merck Life Science UK 2020 Safety Data Sheet provided to the European Chemicals Agency's Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH)—a 2006 European Union regulation, ethylene oxide is "presumed to have carcinogenic potential for humans."
References
Cited sources
*
External links
EOSA Promoting the safe use of Ethylene Oxide for SterilizationWebBook page for C2H4ONational Institute for Occupational Safety and Health – Ethylene Oxide Topic Page
{{Authority control
Biocides
Chemical hazards
Commodity chemicals
Epoxides
Explosive chemicals
Explosive gases
Fungicides
Gaseous signaling molecules
IARC Group 1 carcinogens
Monomers
Organic compounds with 2 carbon atoms
Suspected testicular toxicants

 The epoxy cycle of ethylene oxide is an almost regular triangle with bond angles of about 60° and a significant angular strain corresponding to the energy of 105 kJ/mol. For comparison, in
The epoxy cycle of ethylene oxide is an almost regular triangle with bond angles of about 60° and a significant angular strain corresponding to the energy of 105 kJ/mol. For comparison, in  and more specific reactions are described below.
and more specific reactions are described below.


 The reaction mechanism is as follows:
:
The reaction mechanism is as follows:
:  The dimerization reaction is unselective. By-products include
The dimerization reaction is unselective. By-products include  Reaction of phosphorus pentachloride with ethylene oxide produces ethylene dichloride:
: (CH2CH2)O + PCl5 → Cl–CH2CH2–Cl + POCl3
Other dichloro derivatives of ethylene oxide can be obtained by combined action of sulfuryl chloride (SOCl2) and pyridine and of triphenylphosphine and carbon tetrachloride.
Phosphorus trichloride reacts with ethylene oxide forming chloroethyl esters of phosphorous acid:
: (CH2CH2)O + PCl3 → Cl–CH2CH2–OPCl2
: 2 (CH2CH2)O + PCl3 → (Cl–CH2CH2–O)2PCl
: 3 (CH2CH2)O + PCl3 → Cl–CH2CH2–O)3P
The reaction product of ethylene oxide with acyl chlorides in the presence of sodium iodide is a complex iodoethyl ester:
: (CH2CH2)O + RCOCl + NaI → RC(O)–OCH2CH2–I + NaCl
Heating ethylene oxide to 100 °C with
Reaction of phosphorus pentachloride with ethylene oxide produces ethylene dichloride:
: (CH2CH2)O + PCl5 → Cl–CH2CH2–Cl + POCl3
Other dichloro derivatives of ethylene oxide can be obtained by combined action of sulfuryl chloride (SOCl2) and pyridine and of triphenylphosphine and carbon tetrachloride.
Phosphorus trichloride reacts with ethylene oxide forming chloroethyl esters of phosphorous acid:
: (CH2CH2)O + PCl3 → Cl–CH2CH2–OPCl2
: 2 (CH2CH2)O + PCl3 → (Cl–CH2CH2–O)2PCl
: 3 (CH2CH2)O + PCl3 → Cl–CH2CH2–O)3P
The reaction product of ethylene oxide with acyl chlorides in the presence of sodium iodide is a complex iodoethyl ester:
: (CH2CH2)O + RCOCl + NaI → RC(O)–OCH2CH2–I + NaCl
Heating ethylene oxide to 100 °C with  In industry, a similar reaction is carried out at high pressure and temperature in the presence of quaternary ammonium or phosphonium salts as a catalyst.
Reaction of ethylene oxide with formaldehyde at 80–150 °C in the presence of a catalyst leads to the formation of dioxolane, 1,3-dioxolane:
:
In industry, a similar reaction is carried out at high pressure and temperature in the presence of quaternary ammonium or phosphonium salts as a catalyst.
Reaction of ethylene oxide with formaldehyde at 80–150 °C in the presence of a catalyst leads to the formation of dioxolane, 1,3-dioxolane:
:  Substituting formaldehyde by other aldehydes or ketones results in a 2-substituted 1,3-dioxolane (yield: 70–85%, catalyst: tetraethylammonium bromide).
Catalytic hydroformylation of ethylene oxide gives hydroxypropanal which can be hydrogenated to propane-1,3-diol:
:
Substituting formaldehyde by other aldehydes or ketones results in a 2-substituted 1,3-dioxolane (yield: 70–85%, catalyst: tetraethylammonium bromide).
Catalytic hydroformylation of ethylene oxide gives hydroxypropanal which can be hydrogenated to propane-1,3-diol:
:  Oxidation by peroxy acids is efficient for higher alkenes, but not for ethylene. The above reaction is slow and has low yield, therefore it is not used in the industry.
Oxidation by peroxy acids is efficient for higher alkenes, but not for ethylene. The above reaction is slow and has low yield, therefore it is not used in the industry.

 Ethylene oxide is one of the most important raw materials used in large-scale chemical production. Most ethylene oxide is used for synthesis of ethylene glycols, including diethylene glycol and triethylene glycol, that accounts for up to 75% of global consumption. Other important products include ethylene glycol ethers, ethanolamines, and ethoxylates. Among glycols, ethylene glycol is used as antifreeze, in the production of polyester and polyethylene terephthalate (PET – raw material for plastic bottles), liquid coolants, and solvents.
Polyethyleneglycols are used in perfumes, cosmetics, pharmaceuticals, lubricants, paint thinners, and plasticizers. Ethylene glycol ethers are part of brake fluids, detergents, solvents, lacquers, and paints. Ethanolamines are used in the manufacture of soap and detergents and for purification of natural gas. Ethoxylates are reaction products of ethylene oxide with higher alcohols, acids, or amines. They are used in the manufacture of detergents, surfactants, emulsifiers, and dispersants.
Whereas synthesis of ethylene glycols is the major application of ethylene oxide, its percentage varies greatly depending on the region: from 44% in the Western Europe, 63% in Japan, and 73% in North America to 90% in the rest of Asia, and 99% in Africa.
Ethylene oxide is one of the most important raw materials used in large-scale chemical production. Most ethylene oxide is used for synthesis of ethylene glycols, including diethylene glycol and triethylene glycol, that accounts for up to 75% of global consumption. Other important products include ethylene glycol ethers, ethanolamines, and ethoxylates. Among glycols, ethylene glycol is used as antifreeze, in the production of polyester and polyethylene terephthalate (PET – raw material for plastic bottles), liquid coolants, and solvents.
Polyethyleneglycols are used in perfumes, cosmetics, pharmaceuticals, lubricants, paint thinners, and plasticizers. Ethylene glycol ethers are part of brake fluids, detergents, solvents, lacquers, and paints. Ethanolamines are used in the manufacture of soap and detergents and for purification of natural gas. Ethoxylates are reaction products of ethylene oxide with higher alcohols, acids, or amines. They are used in the manufacture of detergents, surfactants, emulsifiers, and dispersants.
Whereas synthesis of ethylene glycols is the major application of ethylene oxide, its percentage varies greatly depending on the region: from 44% in the Western Europe, 63% in Japan, and 73% in North America to 90% in the rest of Asia, and 99% in Africa.
 Ethylene oxide is one of the most commonly used sterilization methods in the healthcare industry because of its non-damaging effects for delicate instruments and devices that require sterilization, and for its wide range of material compatibility. It is used for instruments that cannot tolerate heat, moisture, or abrasive chemicals, such as electronics, optical equipment, paper, rubber, and plastics. It was developed in the 1940s as a sterilant by the US military, and its use as a medical sterilant dates to the late 1950s, when the McDonald process was patented for medical devices. The Anprolene system was patented in the 1960s by Andersen Products, and it remains the most commonly used system in several niche markets, notably the veterinary market and some international markets. It relies on the use of a flexible sterilization chamber and an EtO cartridge for small volume sterilization, and where environmental and/or portability considerations dictate the use of a low dose. It is therefore referred to as the "flexible chamber sterilization" method, or the "gas diffusion sterilization" method.
In the United States, the operation of EtO sterilization is overseen by the EPA through the National Emissions Standards for Hazardous Air Pollutants (NESHAP).
Ethylene oxide is one of the most commonly used sterilization methods in the healthcare industry because of its non-damaging effects for delicate instruments and devices that require sterilization, and for its wide range of material compatibility. It is used for instruments that cannot tolerate heat, moisture, or abrasive chemicals, such as electronics, optical equipment, paper, rubber, and plastics. It was developed in the 1940s as a sterilant by the US military, and its use as a medical sterilant dates to the late 1950s, when the McDonald process was patented for medical devices. The Anprolene system was patented in the 1960s by Andersen Products, and it remains the most commonly used system in several niche markets, notably the veterinary market and some international markets. It relies on the use of a flexible sterilization chamber and an EtO cartridge for small volume sterilization, and where environmental and/or portability considerations dictate the use of a low dose. It is therefore referred to as the "flexible chamber sterilization" method, or the "gas diffusion sterilization" method.
In the United States, the operation of EtO sterilization is overseen by the EPA through the National Emissions Standards for Hazardous Air Pollutants (NESHAP).