Xenon Compounds on:
[Wikipedia]
[Google]
[Amazon]
Xenon compounds are compounds containing the element
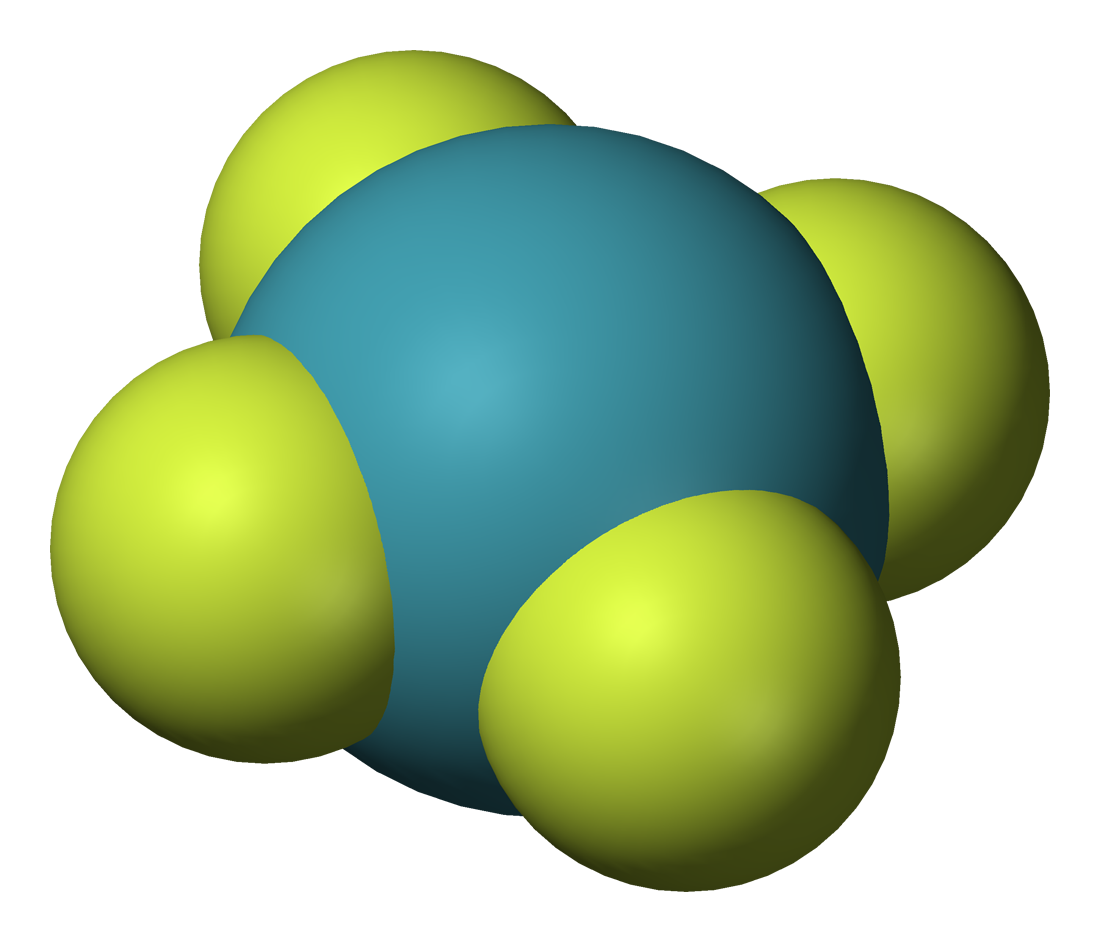
 Three
Three
xenon
Xenon is a chemical element; it has symbol Xe and atomic number 54. It is a dense, colorless, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as the ...
(Xe). After Neil Bartlett's discovery in 1962 that xenon can form chemical compounds, a large number of xenon compounds have been discovered and described. Almost all known xenon compounds contain the electronegative
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
atoms fluorine or oxygen. The chemistry of xenon in each oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons ...
is analogous to that of the neighboring element iodine
Iodine is a chemical element; it has symbol I and atomic number 53. The heaviest of the stable halogens, it exists at standard conditions as a semi-lustrous, non-metallic solid that melts to form a deep violet liquid at , and boils to a vi ...
in the immediately lower oxidation state.
Halides

 Three
Three fluoride
Fluoride (). According to this source, is a possible pronunciation in British English. is an Inorganic chemistry, inorganic, Monatomic ion, monatomic Ion#Anions and cations, anion of fluorine, with the chemical formula (also written ), whose ...
s are known: , , and . XeF is theorized to be unstable. These are the starting points for the synthesis of almost all xenon compounds.
The solid, crystalline difluoride is formed when a mixture of fluorine
Fluorine is a chemical element; it has Chemical symbol, symbol F and atomic number 9. It is the lightest halogen and exists at Standard temperature and pressure, standard conditions as pale yellow Diatomic molecule, diatomic gas. Fluorine is extre ...
and xenon gases is exposed to ultraviolet light. The ultraviolet component of ordinary daylight is sufficient. Long-term heating of at high temperatures under an catalyst yields . Pyrolysis
Pyrolysis is a process involving the Bond cleavage, separation of covalent bonds in organic matter by thermal decomposition within an Chemically inert, inert environment without oxygen. Etymology
The word ''pyrolysis'' is coined from the Gree ...
of in the presence of NaF yields high-purity .
The xenon fluorides behave as both fluoride acceptors and fluoride donors, forming salts that contain such cations as and , and anions such as , , and . The green, paramagnetic is formed by the reduction of by xenon gas.
also forms coordination complexes
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or ...
with transition metal ions. More than 30 such complexes have been synthesized and characterized.
Whereas the xenon fluorides are well characterized, the other halides are not. Xenon dichloride, formed by the high-frequency irradiation of a mixture of xenon, fluorine, and silicon
Silicon is a chemical element; it has symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid (sometimes considered a non-metal) and semiconductor. It is a membe ...
or carbon tetrachloride
Carbon tetrachloride, also known by many other names (such as carbon tet for short and tetrachloromethane, also IUPAC nomenclature of inorganic chemistry, recognised by the IUPAC), is a chemical compound with the chemical formula CCl4. It is a n ...
, is reported to be an endothermic, colorless, crystalline compound that decomposes into the elements at 80 °C. However, may be merely a van der Waals molecule
A van der Waals molecule is a weakly bound complex of atoms or molecules held together by intermolecular attractions such as van der Waals forces or by hydrogen bonds.
The name originated in the beginning of the 1970s when stable molecular clust ...
of weakly bound Xe atoms and molecules and not a real compound. Theoretical calculations indicate that the linear molecule is less stable than the van der Waals complex. Xenon tetrachloride and xenon dibromide
Xenon dibromide is an unstable chemical compound with the chemical formula XeBr2. It was only produced by the decomposition of iodine-129:
:129IBr2– → XeBr2 + e–
Attempts to prepare this compound by combining elemental xenon and bromine only ...
are more unstable that they cannot be synthesized by chemical reactions. They were created by radioactive decay of and , respectively.
Oxides and oxohalides
Three oxides of xenon are known: xenon trioxide () and xenon tetroxide (), both of which are dangerously explosive and powerful oxidizing agents, andxenon dioxide
Xenon dioxide, or xenon(IV) oxide, is a compound of xenon and oxygen with formula XeO2 which was synthesized in 2011. It is synthesized at 0 °C by hydrolysis of xenon tetrafluoride in aqueous sulfuric acid:
:
Structure
has an extended (c ...
(XeO2), which was reported in 2011 with a coordination number
In chemistry, crystallography, and materials science, the coordination number, also called ligancy, of a central atom in a molecule or crystal is the number of atoms, molecules or ions bonded to it. The ion/molecule/atom surrounding the central ion ...
of four. XeO2 forms when xenon tetrafluoride is poured over ice. Its crystal structure may allow it to replace silicon in silicate minerals. The XeOO+ cation has been identified by infrared spectroscopy
Infrared spectroscopy (IR spectroscopy or vibrational spectroscopy) is the measurement of the interaction of infrared radiation with matter by absorption, emission, or reflection. It is used to study and identify chemical substances or functio ...
in solid argon
Argon is a chemical element; it has symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abu ...
.
Xenon does not react with oxygen directly; the trioxide is formed by the hydrolysis of :
: + 3 → + 6 HF
is weakly acidic, dissolving in alkali to form unstable ''xenate'' salts containing the anion. These unstable salts easily disproportionate into xenon gas and perxenate salts, containing the anion.
Barium perxenate, when treated with concentrated sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
, yields gaseous xenon tetroxide:
: + 2 → 2 + 2 +
To prevent decomposition, the xenon tetroxide thus formed is quickly cooled into a pale-yellow solid. It explodes above −35.9 °C into xenon and oxygen gas, but is otherwise stable.
A number of xenon oxyfluorides are known, including , , , and . is formed by reacting with xenon gas at low temperatures. It may also be obtained by partial hydrolysis of . It disproportionates at −20 °C into and . is formed by the partial hydrolysis of ...
: + → + 2
...or the reaction of with sodium perxenate, . The latter reaction also produces a small amount of .
is also formed by partial hydrolysis of .
: + 2 → + 4
reacts with CsF to form the anion, while XeOF3 reacts with the alkali metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names ...
fluorides KF, RbF and CsF to form the anion.
Other compounds
Xenon can be directly bonded to a less electronegative element than fluorine or oxygen, particularlycarbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
. Electron-withdrawing groups, such as groups with fluorine substitution, are necessary to stabilize these compounds. Numerous such compounds have been characterized, including:
* , where C6F5 is the pentafluorophenyl group.
*
*
*
*
*
*
*
*
Other compounds containing xenon bonded to a less electronegative element include and . The latter is synthesized from dioxygenyl
The dioxygenyl ion, , has been studied in both the gas phase and in salts with anions that cannot be oxidized. The first synthesis was []. Rather than the triple bond of , the bond order is considered to be . Relative to most molecules, this ioni ...
tetrafluoroborate, , at −100 °C.
An unusual ion containing xenon is the tetraxenonogold(II)
Tetraxenonogold(II), gold tetraxenide(II) or AuXe is a cationic complex consisting of a central gold atom surrounded by four xenon atoms. It is a covalent complex with a square planar configuration of atoms. The complex is found in the compound A ...
cation, , which contains Xe–Au bonds.
This ion occurs in the compound , and is remarkable in having direct chemical bonds between two notoriously unreactive atoms, xenon and gold
Gold is a chemical element; it has chemical symbol Au (from Latin ) and atomic number 79. In its pure form, it is a brightness, bright, slightly orange-yellow, dense, soft, malleable, and ductile metal. Chemically, gold is a transition metal ...
, with xenon acting as a transition metal ligand. A similar mercury complex (HgXe)(Sb3F17) (formulated as gXe2+Sb2F11–] bF6– is also known. Xenon reversibly complexes gaseous M(CO)5, where M=Cr, Mo, or W. ''p''-block metals also bind noble gases: XeBeO has been observed spectroscopically and both XeBeS and FXeBO are predicted stable.
The compound contains a Xe–Xe bond, the longest element-element bond known (308.71 pm = 3.0871 Å).
In 1995, M. Räsänen and co-workers, scientists at the University of Helsinki
The University of Helsinki (, ; UH) is a public university in Helsinki, Finland. The university was founded in Turku in 1640 as the Royal Academy of Åbo under the Swedish Empire, and moved to Helsinki in 1828 under the sponsorship of Alexander ...
in Finland
Finland, officially the Republic of Finland, is a Nordic country in Northern Europe. It borders Sweden to the northwest, Norway to the north, and Russia to the east, with the Gulf of Bothnia to the west and the Gulf of Finland to the south, ...
, announced the preparation of xenon dihydride (HXeH), and later xenon hydride-hydroxide (HXeOH), hydroxenoacetylene (HXeCCH), and other Xe-containing molecules. In 2008, Khriachtchev ''et al.'' reported the preparation of HXeOXeH by the photolysis
Photodissociation, photolysis, photodecomposition, or photofragmentation is a chemical reaction in which molecules of a chemical compound are broken down by absorption of light or photons. It is defined as the interaction of one or more photons wi ...
of water within a cryogenic
In physics, cryogenics is the production and behaviour of materials at very low temperatures.
The 13th International Institute of Refrigeration's (IIR) International Congress of Refrigeration (held in Washington, DC in 1971) endorsed a univers ...
xenon matrix. Deuterated molecules, HXeOD and DXeOH, have also been produced.
Clathrates and excimers
In addition to compounds where xenon forms achemical bond
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds or through the sharing of electrons a ...
, xenon can form clathrate
A clathrate is a chemical substance consisting of a lattice (group), lattice that traps or contains molecules. The word ''clathrate'' is derived from the Latin language, Latin (), meaning 'with bars, Crystal structure, latticed'. Most clathrate ...
s—substances where xenon atoms or pairs are trapped by the crystalline lattice
In geometry and crystallography, a Bravais lattice, named after , is an infinite array of discrete points generated by a set of discrete translation operations described in three dimensional space by
: \mathbf = n_1 \mathbf_1 + n_2 \mathbf_2 ...
of another compound. One example is xenon hydrate (Xe·H2O), where xenon atoms occupy vacancies in a lattice of water molecules. This clathrate has a melting point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state of matter, state from solid to liquid. At the melting point the solid and liquid phase (matter), phase exist in Thermodynamic equilib ...
of 24 °C. The deuterated version of this hydrate has also been produced. Another example is xenon hydride
In chemistry, a hydride is formally the anion of hydrogen (H−), a hydrogen ion with two electrons. In modern usage, this is typically only used for ionic bonds, but it is sometimes (and has been more frequently in the past) applied to all che ...
(Xe(H2)8), in which xenon pairs ( dimers) are trapped inside solid hydrogen
Solid hydrogen is the solid state of the element hydrogen. At standard pressure, this is achieved by decreasing the temperature below hydrogen's melting point of . It was collected for the first time by James Dewar in 1899 and published with the ...
. Such clathrate hydrate
Clathrate hydrates, or gas hydrates, clathrates, or hydrates, are crystalline water-based solids physically resembling ice, in which small non-polar molecules (typically gases) or polar molecules with large hydrophobic moieties are trapped ins ...
s can occur naturally under conditions of high pressure, such as in Lake Vostok
Lake Vostok () is the largest of Antarctica's 675 known subglacial lakes. Lake Vostok is located at the southern Pole of Cold, beneath Russia's Vostok Station under the surface of the central East Antarctic Ice Sheet, which is at above mean se ...
underneath the Antarctic
The Antarctic (, ; commonly ) is the polar regions of Earth, polar region of Earth that surrounds the South Pole, lying within the Antarctic Circle. It is antipodes, diametrically opposite of the Arctic region around the North Pole.
The Antar ...
ice sheet. Clathrate formation can be used to fractionally distill xenon, argon and krypton.
Xenon can also form endohedral fullerene
Endohedral fullerenes, also called endofullerenes, are fullerenes that have additional atoms, ions, or clusters enclosed within their inner spheres. The first lanthanum C60 complex called La@C60 was synthesized in 1985. The @ (at sign) in t ...
compounds, where a xenon atom is trapped inside a fullerene
A fullerene is an allotropes of carbon, allotrope of carbon whose molecules consist of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to six atoms. The molecules may ...
molecule. The xenon atom trapped in the fullerene can be observed by 129Xe nuclear magnetic resonance
Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are disturbed by a weak oscillating magnetic field (in the near field) and respond by producing an electromagnetic signal with a ...
(NMR) spectroscopy. Through the sensitive chemical shift
In nuclear magnetic resonance (NMR) spectroscopy, the chemical shift is the resonant frequency of an atomic nucleus relative to a standard in a magnetic field. Often the position and number of chemical shifts are diagnostic of the structure of ...
of the xenon atom to its environment, chemical reactions on the fullerene molecule can be analyzed. These observations are not without caveat, however, because the xenon atom has an electronic influence on the reactivity of the fullerene.
When xenon atoms are in the ground energy state, they repel each other and will not form a bond. When xenon atoms becomes energized, however, they can form an excimer
An excimer (originally short for excited dimer) is a short-lived polyatomic molecule formed from two species that do not form a stable molecule in the ground state. In this case, formation of molecules is possible only if such atom is in an elec ...
(excited dimer) until the electrons return to the ground state
The ground state of a quantum-mechanical system is its stationary state of lowest energy; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state ...
. This entity is formed because the xenon atom tends to complete the outermost electronic shell by adding an electron from a neighboring xenon atom. The typical lifetime of a xenon excimer is 1–5 nanoseconds, and the decay releases photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless particles that can ...
s with wavelength
In physics and mathematics, wavelength or spatial period of a wave or periodic function is the distance over which the wave's shape repeats.
In other words, it is the distance between consecutive corresponding points of the same ''phase (waves ...
s of about 150 and 173 nm. Xenon can also form excimers with other elements, such as the halogen
The halogens () are a group in the periodic table consisting of six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and the radioactive elements astatine (At) and tennessine (Ts), though some authors would ...
s bromine
Bromine is a chemical element; it has chemical symbol, symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between th ...
, chlorine
Chlorine is a chemical element; it has Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between ...
, and fluorine
Fluorine is a chemical element; it has Chemical symbol, symbol F and atomic number 9. It is the lightest halogen and exists at Standard temperature and pressure, standard conditions as pale yellow Diatomic molecule, diatomic gas. Fluorine is extre ...
.
References
{{Xenon compounds Xenon compounds Chemical compounds by element Explosive chemicals