|
Xenon Compounds
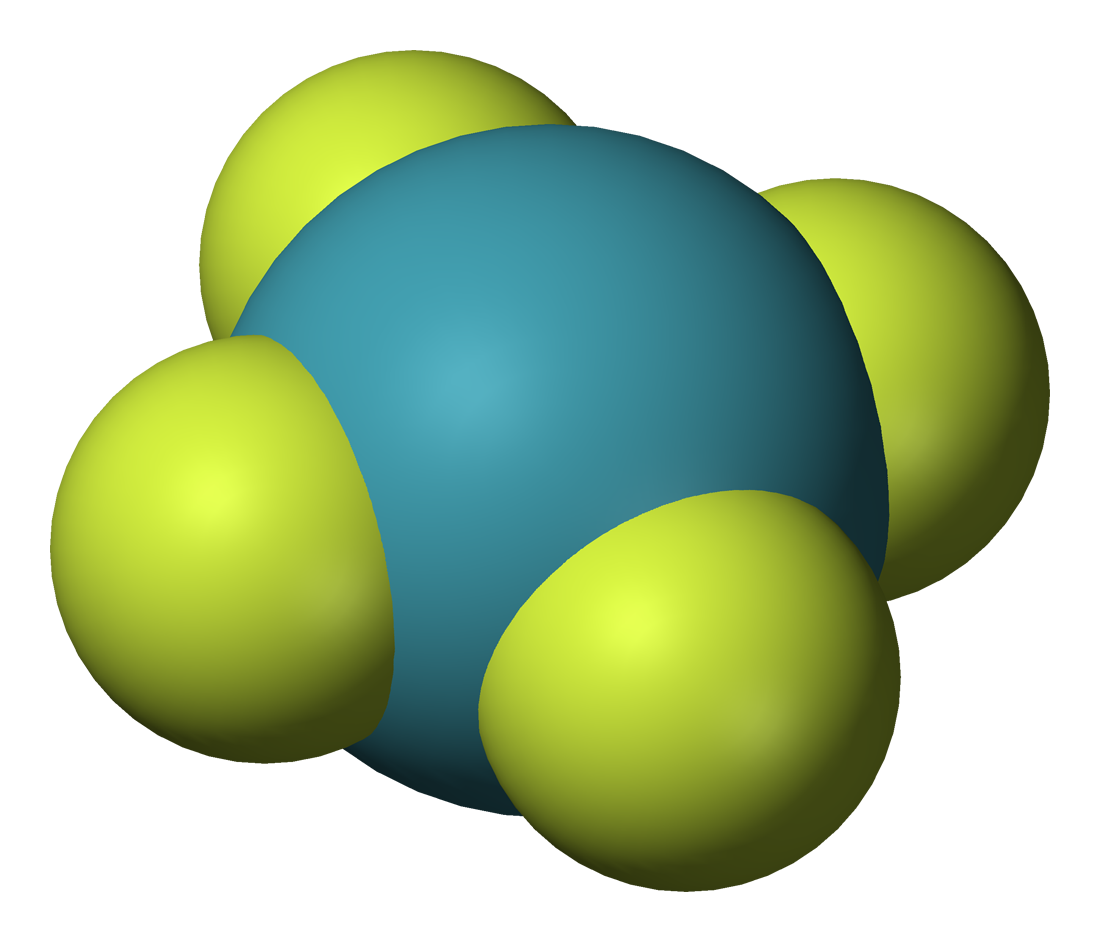
Xenon compounds are compounds containing the element xenon (Xe). After Neil Bartlett's discovery in 1962 that xenon can form chemical compounds, a large number of xenon compounds have been discovered and described. Almost all known xenon compounds contain the electronegative atoms fluorine or oxygen. The chemistry of xenon in each oxidation state is analogous to that of the neighboring element iodine in the immediately lower oxidation state. Halides Three fluorides are known: , , and . XeF is theorized to be unstable. These are the starting points for the synthesis of almost all xenon compounds. The solid, crystalline difluoride is formed when a mixture of fluorine and xenon gases is exposed to ultraviolet light. The ultraviolet component of ordinary daylight is sufficient. Long-term heating of at high temperatures under an catalyst yields . Pyrolysis of in the presence of NaF yields high-purity . The xenon fluorides behave as both fluoride acceptors and fluoride dono ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Xenon
Xenon is a chemical element; it has symbol Xe and atomic number 54. It is a dense, colorless, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as the formation of xenon hexafluoroplatinate, the first noble gas compound to be synthesized. Xenon is used in flash lamps and arc lamps, and as a general anesthetic. The first excimer laser design used a xenon dimer molecule (Xe2) as the lasing medium, and the earliest laser designs used xenon flash lamps as pumps. Xenon is also used to search for hypothetical weakly interacting massive particles and as a propellant for ion thrusters in spacecraft. Naturally occurring xenon consists of seven stable isotopes and two long-lived radioactive isotopes. More than 40 unstable xenon isotopes undergo radioactive decay, and the isotope ratios of xenon are an important tool for studying the early history of the Solar System. Radioactive xe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Tetrachloride
Carbon tetrachloride, also known by many other names (such as carbon tet for short and tetrachloromethane, also IUPAC nomenclature of inorganic chemistry, recognised by the IUPAC), is a chemical compound with the chemical formula CCl4. It is a non-flammable, dense, colourless liquid with a "sweet" chloroform-like odour that can be detected at low levels. It was formerly widely used in fire extinguishers, as a precursor to refrigerants, an anthelmintic and a cleaning agent, but has since been phased out because of environmental and safety concerns. Exposure to high concentrations of carbon tetrachloride can affect the central nervous system and degenerate the liver and kidneys. Prolonged exposure can be fatal. Properties In the carbon tetrachloride molecule, four chlorine atoms are positioned symmetrically as corners in a tetrahedron, tetrahedral configuration joined to a central carbon atom by single covalent bonds. Because of this symmetric geometry, CCl4 is non-polar. methane, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disproportionation
In chemistry, disproportionation, sometimes called dismutation, is a redox reaction in which one compound of intermediate oxidation state converts to two compounds, one of higher and one of lower oxidation state. The reverse of disproportionation, such as when a compound in an intermediate oxidation state is formed from precursors of lower and higher oxidation states, is called ''comproportionation'', also known as ''symproportionation''. More generally, the term can be applied to any desymmetrizing reaction where two molecules of one type react to give one each of two different types: : This expanded definition is not limited to redox reactions, but also includes some molecular autoionization reactions, such as the self-ionization of water. In contrast, some authors use the term ''redistribution'' to refer to reactions of this type (in either direction) when only ligand exchange but no redox is involved and distinguish such processes from disproportionation and comproportionati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Argon
Argon is a chemical element; it has symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abundant as water vapor (which averages about 4000 ppmv, but varies greatly), 23 times as abundant as carbon dioxide (400 ppmv), and more than 500 times as abundant as neon (18 ppmv). Argon is the most abundant noble gas in Earth's crust, comprising 0.00015% of the crust. Nearly all argon in Earth's atmosphere is radiogenic argon-40, derived from the decay of potassium-40 in Earth's crust. In the universe, argon-36 is by far the most common argon isotope, as it is the most easily produced by stellar nucleosynthesis in supernovas. The name "argon" is derived from the Greek word , neuter singular form of meaning 'lazy' or 'inactive', as a reference to the fact that the element undergoes almost no chemical reactions. The complete oc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Infrared Spectroscopy
Infrared spectroscopy (IR spectroscopy or vibrational spectroscopy) is the measurement of the interaction of infrared radiation with matter by absorption, emission, or reflection. It is used to study and identify chemical substances or functional groups in solid, liquid, or gaseous forms. It can be used to characterize new materials or identify and verify known and unknown samples. The method or technique of infrared spectroscopy is conducted with an instrument called an infrared spectrometer (or spectrophotometer) which produces an infrared spectrum. An IR spectrum can be visualized in a graph of infrared light absorbance (or transmittance) on the vertical axis vs. frequency, wavenumber or wavelength on the horizontal axis. Typical units of wavenumber used in IR spectra are reciprocal centimeters, with the symbol cm−1. Units of IR wavelength are commonly given in micrometers (formerly called "microns"), symbol μm, which are related to the wavenumber in a reciprocal way ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Journal Of The American Chemical Society
The ''Journal of the American Chemical Society'' (also known as JACS) is a weekly peer-reviewed scientific journal that was established in 1879 by the American Chemical Society. The journal has absorbed two other publications in its history, the ''Journal of Analytical and Applied Chemistry'' (July 1893) and the ''American Chemical Journal'' (January 1914). It covers all fields of chemistry. Since 2021, the editor-in-chief is Erick M. Carreira (ETH Zurich). In 2014, the journal moved to a hybrid open access publishing model. Abstracting and indexing The journal is abstracted and indexed in: According to the ''Journal Citation Reports'', the journal has a 2023 impact factor The impact factor (IF) or journal impact factor (JIF) of an academic journal is a type of journal ranking. Journals with higher impact factor values are considered more prestigious or important within their field. The Impact Factor of a journa ... of 14.4. Editors-in-chief The following people are or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coordination Number
In chemistry, crystallography, and materials science, the coordination number, also called ligancy, of a central atom in a molecule or crystal is the number of atoms, molecules or ions bonded to it. The ion/molecule/atom surrounding the central ion/molecule/atom is called a ligand. This number is determined somewhat differently for molecules than for crystals. For molecules and polyatomic ions the coordination number of an atom is determined by simply counting the other atoms to which it is bonded (by either single or multiple bonds). For example, [Cr(NH3)2Cl2Br2]− has Cr3+ as its central cation, which has a coordination number of 6 and is described as ''hexacoordinate''. The common coordination numbers are 4, 6 and 8. Molecules, polyatomic ions and coordination complexes In chemistry, coordination number, defined originally in 1893 by Alfred Werner, is the total number of neighbors of a central atom in a molecule or ion. The concept is most commonly applied to coordination ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Xenon Dioxide
Xenon dioxide, or xenon(IV) oxide, is a compound of xenon and oxygen with formula XeO2 which was synthesized in 2011. It is synthesized at 0 °C by hydrolysis of xenon tetrafluoride in aqueous sulfuric acid: : Structure has an extended (chain or network) structure in which xenon and oxygen have coordination numbers of four and two respectively. The geometry at xenon is square planar, consistent with VSEPR theory for four ligands and two lone pairs (or AX4E2 in the notation of VSEPR theory). The XeO2 network does not share a crystal structure of SiO2 (which has tetrahedral coordination at Si), but XeO2 units are believed to intermix with SiO2 in Earth's mantle. Computational studies suggest that xenon cannot displace silicon directly, but can fill pre-existing silicon vacancies. The stability of the resulting material under standard conditions depends on its allotrope. Patterned off quartz, it likely decomposes; but materials patterned off fibrous silica may be metast ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
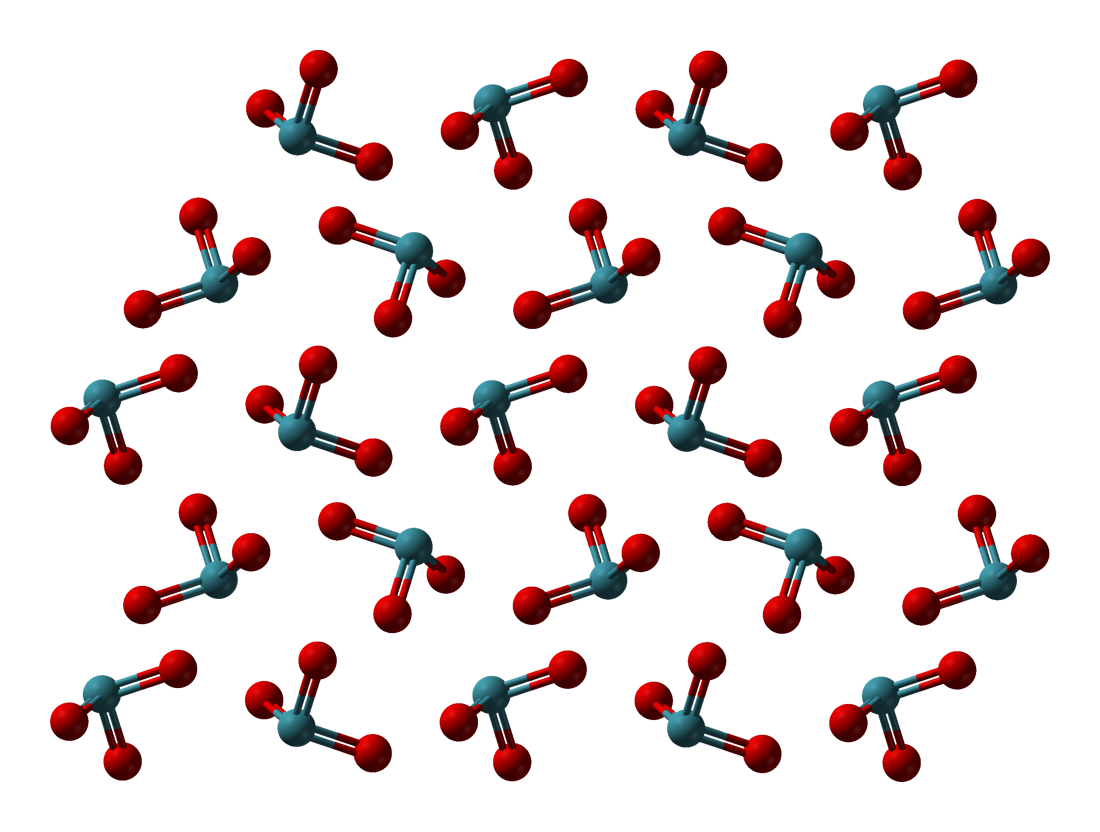
Xenon Tetroxide
Xenon tetroxide is a chemical compound of xenon and oxygen with molecular formula XeO4, remarkable for being a relatively stable compound of a noble gas. It is a yellow crystalline solid that is stable below −35.9 ° C; above that temperature it is very prone to exploding and decomposing into elemental xenon and oxygen (O2). All eight valence electrons of xenon are involved in the bonds with the oxygen, and the oxidation state of the xenon atom is +8. Oxygen is the only element that can bring xenon up to its highest oxidation state; even fluorine can only give XeF6 (+6). Two other short-lived xenon compounds with an oxidation state of +8, XeO3F2 and XeO2F4, are accessible by the reaction of xenon tetroxide with xenon hexafluoride. XeO3F2 and XeO2F4 can be detected with mass spectrometry. The perxenates are also compounds where xenon has the +8 oxidation state. __TOC__ Reactions At temperatures above −35.9 °C, xenon tetroxide is very prone to explosion, de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Xenon Trioxide
Xenon trioxide is an unstable compound of xenon in its +6 oxidation state. It is a very powerful oxidizing agent, and liberates oxygen from water slowly, accelerated by exposure to sunlight. It is dangerously explosive upon contact with organic materials. When it detonates, it releases xenon and oxygen gas. Chemistry Synthesis of xenon trioxide is by aqueous hydrolysis of : : + 3 → + 6 HF The resulting xenon trioxide crystals are a strong oxidising agent and can oxidise most substances that are at all oxidisable. However, it is slow-acting and this reduces its usefulness. Above 25 °C, xenon trioxide is very prone to violent explosion: :2 XeO3 → 2 Xe + 3 O2 (Δ''H''f = −403 kJ/ mol) When it dissolves in water, an acidic solution of xenic acid is formed: :XeO3(aq) + H2O → H2XeO4 H+ + This solution is stable at room temperature and lacks the explosive properties of xenon trioxide. It oxidises carboxylic acids quantitatively to carbon dioxide and water. Al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decay Technique
In chemistry, the decay technique is a method to generate chemical species such as radicals, carbocations, and other potentially unstable covalent structures by radioactive decay of other compounds. For example, decay of a tritium-labeled molecule yields an ionized helium atom, which might then break off to leave a cationic molecular fragment. The technique was developed in 1963 by the Italian chemist Fulvio Cacace at the University of Rome. It has allowed the study of a vast number of otherwise inaccessible compounds and reactions. It has also provided much of our current knowledge about the chemistry of the helium hydride ion . Carbocation generation In the basic method, a molecule is prepared where the vacant bond of the desired radical or ion is satisfied by an atom of tritium , the radioactive isotope of hydrogen with mass number 3. As the tritium undergoes beta decay (with a half-life of 12.32 years), it is transformed into an ion of helium-3, creating the cation . In th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Xenon Dibromide
Xenon dibromide is an unstable chemical compound with the chemical formula XeBr2. It was only produced by the decomposition of iodine-129: :129IBr2– → XeBr2 + e– Attempts to prepare this compound by combining elemental xenon and bromine only resulted in the XeBr radical. This compound is expected to be less stable than xenon difluoride Xenon is a chemical element; it has symbol Xe and atomic number 54. It is a dense, colorless, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as th ... and xenon dichloride. It is also expected to decompose to xenon and bromine. References {{Bromides Xenon(II) compounds Bromides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |