Vinyl Iodide Functional Group on:
[Wikipedia]
[Google]
[Amazon]
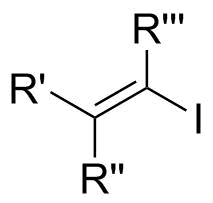 In
In
 In cross- coupling reactions, typically vinyl iodides react faster and under more mild conditions than vinyl chloride and vinyl bromide. The order of reactivity is based on the strength of carbon-halogen bond. C-I bond is the weakest of the halogens, the
In cross- coupling reactions, typically vinyl iodides react faster and under more mild conditions than vinyl chloride and vinyl bromide. The order of reactivity is based on the strength of carbon-halogen bond. C-I bond is the weakest of the halogens, the 
 Besides using vinyl iodides as useful substrates in transition metal cross-
Besides using vinyl iodides as useful substrates in transition metal cross-
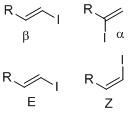 Vinyl iodides are synthesized by methods such as
Vinyl iodides are synthesized by methods such as
 Another method doesn't involve
Another method doesn't involve 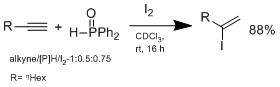 The hydroiodation proceeds by Markovnikov-type adduct, no reaction is observed without addition of hydrophoshine. In a plausible mechanism proposed by Ogawa's group, the hydrophosphine reacts with HI to form an intermediate complex that coordinate HI to do Markovnikov hydroiodation on the alkene. The advantage of this system is the conditions are mild, can tolerate wide range of functional groups.
The hydroiodation proceeds by Markovnikov-type adduct, no reaction is observed without addition of hydrophoshine. In a plausible mechanism proposed by Ogawa's group, the hydrophosphine reacts with HI to form an intermediate complex that coordinate HI to do Markovnikov hydroiodation on the alkene. The advantage of this system is the conditions are mild, can tolerate wide range of functional groups.
 Below is example of employing both Takai olefination and Stork-Zhao olefination in total synthesis of (+)-3-(E)- and (+)-3-(Z)-Pinnatifidenyne.
Below is example of employing both Takai olefination and Stork-Zhao olefination in total synthesis of (+)-3-(E)- and (+)-3-(Z)-Pinnatifidenyne.
 In
In organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
, a vinyl iodide (also known as an iodoalkene) functional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
is an alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
with one or more iodide
An iodide ion is I−. Compounds with iodine in formal oxidation state −1 are called iodides. In everyday life, iodide is most commonly encountered as a component of iodized salt, which many governments mandate. Worldwide, iodine deficiency ...
substituents. Vinyl iodides are versatile molecules that serve as important building blocks and precursors in organic synthesis. They are commonly used in carbon-carbon forming reactions in transition-metal catalyzed cross- coupling reactions, such as Stille reaction
The Stille reaction is a chemical reaction widely used in organic synthesis. The reaction involves the coupling of two organic groups, one of which is carried as an organotin chemistry, organotin compound (also known as organostannanes). A variet ...
, Heck reaction
The Heck reaction (also called the Mizoroki–Heck reaction) is the chemical reaction of an unsaturated halide (or triflate) with an alkene in the presence of a base and a palladium catalyst to form a substituted alkene. It is named after T ...
, Sonogashira coupling
The Sonogashira reaction is a cross-coupling reaction used in organic synthesis to form carbon–carbon bonds. It employs a palladium catalyst as well as copper co-catalyst to form a carbon–carbon bond between a terminal alkyne and an aryl or vi ...
, and Suzuki coupling
The Suzuki reaction or Suzuki coupling is an organic reaction that uses a palladium complex catalyst to cross-couple a boronic acid to an organohalide. It was first published in 1979 by Akira Suzuki, and he shared the 2010 Nobel Prize in Chemis ...
. Synthesis of well-defined geometry or complexity vinyl iodide is important in stereoselective
In chemistry, stereoselectivity is the property of a chemical reaction in which a single reactant forms an unequal mixture of stereoisomers during a non- stereospecific creation of a new stereocenter or during a non-stereospecific transformation o ...
synthesis of natural products
A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical s ...
and drugs
A drug is any chemical substance other than a nutrient or an essential dietary ingredient, which, when administered to a living organism, produces a biological effect. Consumption of drugs can be via inhalation, injection, smoking, ingestio ...
.
Properties
Vinyl iodides are generally stable undernucleophilic
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they a ...
conditions. In SN2 reactions, back-attack is difficult because of steric clash of R groups on carbon adjacent to electrophilic
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carr ...
center (see figure 1a). In addition, the lone pair on iodide donates into the ╥* of the alkene, which reduces electrophilic character on the carbon as a result of decreased positive charge. Also, this stereoelectronic effect
In chemistry, primarily Organic chemistry, organic and computational chemistry, a stereoelectronic effectAlabugin, I. V. Stereoelectronic Effects: the Bridge between Structure and Reactivity. John Wiley & Sons Ltd, Chichester, UK, 2016. http://eu ...
strengthens the C-I bond, thus making removal of the iodide difficult (see figure 1b). In SN1 case, dissociation is difficult because of the strengthened C-I bond and loss of the iodide will generate an unstable carbocation
Carbocation is a general term for ions with a positively charged carbon atom. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further ...
(see figure 1c)
 In cross- coupling reactions, typically vinyl iodides react faster and under more mild conditions than vinyl chloride and vinyl bromide. The order of reactivity is based on the strength of carbon-halogen bond. C-I bond is the weakest of the halogens, the
In cross- coupling reactions, typically vinyl iodides react faster and under more mild conditions than vinyl chloride and vinyl bromide. The order of reactivity is based on the strength of carbon-halogen bond. C-I bond is the weakest of the halogens, the bond dissociation energies
The bond-dissociation energy (BDE, ''D''0, or ''DH°'') is one measure of the strength of a chemical bond . It can be defined as the standard enthalpy change when is cleaved by homolysis to give fragments A and B, which are usually radical s ...
of C-I is 57.6kcal/mol, while fluoride, chloride and bromide are 115, 83.7, 72.1 kcal/mol respectively. As a result of having weaker bond, vinyl iodide does not polymerize as easily as its vinyl halide
In organic chemistry, a vinyl halide is a compound with the formula CH2=CHX (X = halide). The term vinyl group, vinyl is often used to describe any alkenyl group. For this reason, alkenyl halides with the formula RCH=CHX are sometimes called vinyl ...
counterparts, but rather decompose and release iodide
An iodide ion is I−. Compounds with iodine in formal oxidation state −1 are called iodides. In everyday life, iodide is most commonly encountered as a component of iodized salt, which many governments mandate. Worldwide, iodine deficiency ...
.
It is generally believed that vinyl iodide cannot survive common reduction conditions, which reduces the vinyl iodide to an olefin
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as α-olefins.
The International Union of Pu ...
or unsaturated alkane
In organic chemistry, an alkane, or paraffin (a historical trivial name that also has other meanings), is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in whi ...
. However, there is evidence in literature, in which a propargyl alcohol
Propargyl alcohol, or 2-propyn-1-ol, is an organic compound with the formula C3H4O. It is the simplest stable alcohol containing an alkyne functional group. Propargyl alcohol is a colorless viscous liquid that is miscible with water and most pol ...
's alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
was reduced in presence of a vinyl iodide using hydrogen over Pd/CaCO3 or Crabtree's catalyst
Crabtree's catalyst is an organoiridium compound with the formula C5H5N">Tricyclohexylphosphine">P(C6H11)3pyridine">C5H5N/nowiki>PF6. It is a homogeneous catalyst for hydrogenation and hydrogen-transfer reactions, developed by Robert H. Crabtree ...
.

Other applications
 Besides using vinyl iodides as useful substrates in transition metal cross-
Besides using vinyl iodides as useful substrates in transition metal cross-coupling reaction
In organic chemistry, a coupling reaction is a type of reaction in which two reactant molecules are bonded together. Such reactions often require the aid of a metal catalyst. In one important reaction type, a main group organometallic compound o ...
, they can also undergo elimination with a strong base to give corresponding alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
, and they can be converted to suitable vinyl Grignard reagents
Grignard reagents or Grignard compounds are chemical compounds with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromide ...
. Vinyl iodides are converted to Grignard reagents
Grignard reagents or Grignard compounds are chemical compounds with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromide ...
by magnesium-halogen exchange (see Scheme 1a). The scope of this synthetic method is limited since it requires higher temperatures and longer reaction time, which affects functional group tolerance. However, vinyl iodide with electron withdrawing group
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up and down quarks.
E ...
can enhance rate of exchange(see Scheme 1b). Also addition of lithium chloride
Lithium chloride is a chemical compound with the formula Li Cl. The salt is a typical ionic compound (with certain covalent characteristics), although the small size of the Li+ ion gives rise to properties not seen for other alkali metal chlorid ...
helps enhance magnesium-halogen exchange (see Scheme 1c). It is predicted lithium chloride breaks up aggregates in organomagnesium reagents.
Methods of synthesis
 Vinyl iodides are synthesized by methods such as
Vinyl iodides are synthesized by methods such as iodination
In chemistry, halogenation is a chemical reaction which introduces one or more halogens into a chemical compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymers, drugs. ...
and substitution reaction
A substitution reaction (also known as single displacement reaction or single substitution reaction) is a chemical reaction during which one functional group in a chemical compound is replaced by another functional group. Substitution reactions ar ...
. Vinyl iodides with well-defined geometry (regiochemistry
In organic chemistry, regioselectivity is the preference of chemical bonding or breaking in one direction over all other possible directions. It can often apply to which of many possible positions a reagent will affect, such as which proton a str ...
and stereochemistry
Stereochemistry, a subdiscipline of chemistry, studies the spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereoisomers, which are defined ...
) are important in synthesis since many natural products
A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical s ...
and drugs
A drug is any chemical substance other than a nutrient or an essential dietary ingredient, which, when administered to a living organism, produces a biological effect. Consumption of drugs can be via inhalation, injection, smoking, ingestio ...
that have specific structure and dimension. Example of regiochemistry
In organic chemistry, regioselectivity is the preference of chemical bonding or breaking in one direction over all other possible directions. It can often apply to which of many possible positions a reagent will affect, such as which proton a str ...
is whether the iodide is positioned in either alpha or beta position on the olefin. Stereochemistry
Stereochemistry, a subdiscipline of chemistry, studies the spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereoisomers, which are defined ...
such as E-Z notation or cis-trans alkene geometry is important since some transition metal cross- coupling reactions, such as the Suzuki coupling
The Suzuki reaction or Suzuki coupling is an organic reaction that uses a palladium complex catalyst to cross-couple a boronic acid to an organohalide. It was first published in 1979 by Akira Suzuki, and he shared the 2010 Nobel Prize in Chemis ...
, can retain olefin geometry. In synthesis, it is useful to introduce vinyl iodide at various positions to be set up for a coupling reaction at the next synthetic step. Below are various means and methods in introducing and synthesizing vinyl iodides.
Synthesis from alkynes
The common and simplest approach to make vinyl iodide is addition of one equivalent HI toalkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
. This generally makes 2-iodo-1-alkenes or α-vinyl iodide by Markovnikov's rule
In organic chemistry, Markovnikov's rule or Markownikoff's rule describes the outcome of some addition reactions. The rule was formulated by Russian chemist Vladimir Markovnikov in 1870.
Explanation
The rule states that with the addition of a ...
. However, this reaction does not happen at good rates or very high stereoselectively. As a result, most synthetic methods often involve a hydrometalation Hydrometalation (hydrometallation) is a type of chemical reaction in organometallic chemistry in which a chemical compound with a hydrogen to metal bond (M-H, metal hydride) adds to compounds with an unsaturated bond like an alkene (RC=CR) forming ...
step before addition of I+ source.
α-vinyl iodides
Introducing an α-vinyl iodide from a terminal position of an alkyne is a difficult step. in addition, the vinyl metal intermediate can be mildlynucleophilic
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they a ...
, for example vinyl aluminum, can form C-C bonds under catalytic conditions. However, Hoveyda group have demonstrated using nickel-based catalyst (Ni(dppp)Cl2), DIBAL-H with ''N''-iodosuccinimide (NIS), selectively favor α-vinyl iodide with little to no byproducts. Also they observed reverse selectivity for β with Ni(PPh3)2Cl2 in their hydroalumination reactions under same conditions with little or no byproducts. The advantage of this method is that is inexpensive (and commercially available), scalable and one-pot reaction.
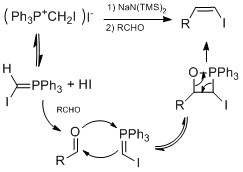
 Another method doesn't involve
Another method doesn't involve hydrometalation Hydrometalation (hydrometallation) is a type of chemical reaction in organometallic chemistry in which a chemical compound with a hydrogen to metal bond (M-H, metal hydride) adds to compounds with an unsaturated bond like an alkene (RC=CR) forming ...
but hydroiodation with I2/hydrophosphine binary system, which was developed by Ogawa's group.
 The hydroiodation proceeds by Markovnikov-type adduct, no reaction is observed without addition of hydrophoshine. In a plausible mechanism proposed by Ogawa's group, the hydrophosphine reacts with HI to form an intermediate complex that coordinate HI to do Markovnikov hydroiodation on the alkene. The advantage of this system is the conditions are mild, can tolerate wide range of functional groups.
The hydroiodation proceeds by Markovnikov-type adduct, no reaction is observed without addition of hydrophoshine. In a plausible mechanism proposed by Ogawa's group, the hydrophosphine reacts with HI to form an intermediate complex that coordinate HI to do Markovnikov hydroiodation on the alkene. The advantage of this system is the conditions are mild, can tolerate wide range of functional groups.
β-vinyl iodides
They are generally more methods in making β-vinyl iodides versus α-vinyl iodides usinghydrometalation Hydrometalation (hydrometallation) is a type of chemical reaction in organometallic chemistry in which a chemical compound with a hydrogen to metal bond (M-H, metal hydride) adds to compounds with an unsaturated bond like an alkene (RC=CR) forming ...
(with aluminum with DIBAL-H ( hydroalumination), with boron (hydroboration
In organic chemistry, hydroboration refers to the addition of a hydrogen-boron bond to certain double and triple bonds involving carbon (, , , and ). This chemical reaction is useful in the organic synthesis of organic compounds.
Hydroboration ...
), with HZrCp2Cl (hydrozirconation
Schwartz's reagent is the common name for the organozirconium compound with the formula (C5H5)2ZrHCl, sometimes called zirconocene hydrochloride or zirconocene chloride hydride, and is named after Jeffrey Schwartz, a chemistry professor at Princeto ...
)). However, hydrometalation Hydrometalation (hydrometallation) is a type of chemical reaction in organometallic chemistry in which a chemical compound with a hydrogen to metal bond (M-H, metal hydride) adds to compounds with an unsaturated bond like an alkene (RC=CR) forming ...
with alkyne with various functional groups often react poorly with side products. The Chong groups have demonstrated using hydrostannation, using Bu3SnH with palladium catalyst with high E stereoselectivity. They observed using sterically bulky ligands gave higher regioselectivity for β-vinyl iodide. The advantage of this technique is this technique can tolerate a wide range of functional groups.
Z selective β-vinyl iodides are slightly more difficult to introduce than E-β-vinyl iodides, often requiring more than one step. Hydroalumination and hydroboration
In organic chemistry, hydroboration refers to the addition of a hydrogen-boron bond to certain double and triple bonds involving carbon (, , , and ). This chemical reaction is useful in the organic synthesis of organic compounds.
Hydroboration ...
usually proceed by syn fashion, therefore selectively favors E geometry. The Oshima group have demonstrated using hydroindation with HInCl selectively favors Z geometry. They suggested that the reaction proceeds by a radical mechanism. They predict that HInCl adds to alkyne by radical addition in a Z geometry. It does not isomerized to E geometry because of low reactivity of radical InCl2 with intermediate complex (no second addition). If second addition occurs then isomerization will occur through diindium intermediate. They confirm a radical mechanism in a mechanistic study with alkyne and alkene cyclization.
Substitution
Substitution is perhaps most useful method in introducing vinyl iodide into the molecule. Halogen-exchange can be useful since vinyl iodides are more reactivity than othervinyl halide
In organic chemistry, a vinyl halide is a compound with the formula CH2=CHX (X = halide). The term vinyl group, vinyl is often used to describe any alkenyl group. For this reason, alkenyl halides with the formula RCH=CHX are sometimes called vinyl ...
s. Buchwald group demonstrates a halogen-exchange from vinyl bromide to vinyl iodide with copper catalyst under mild conditions. It is possible that this method can tolerate various functional groups
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
since these conditions were tested aryl halide
In organic chemistry, an aryl halide (also known as a haloarene) is an aromatic compound in which one or more hydrogen atoms directly bonded to an aromatic ring are replaced by a halide ion (such as fluorine F''−'', chlorine Cl−1,−3,−5, br ...
s initially. The scope of this exchange for regiochemistry
In organic chemistry, regioselectivity is the preference of chemical bonding or breaking in one direction over all other possible directions. It can often apply to which of many possible positions a reagent will affect, such as which proton a str ...
and stereochemistry
Stereochemistry, a subdiscipline of chemistry, studies the spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereoisomers, which are defined ...
is currently unexplored.
Halogen-exchange can also be done with zirconium derivatives that retain olefin
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as α-olefins.
The International Union of Pu ...
’s geometry
The Marek group have further investigated using zirconium catalyst on E or Z vinyl ethers, which selective for E-vinyl ethers. The zirconium's oxophilic
Oxophilicity is the tendency of certain chemical compounds to form oxides by hydrolysis or abstraction of an oxygen atom from another molecule, often from organic compounds. The term is often used to describe metal centers, commonly the early trans ...
nature allows elimination alkoxy
In chemistry, the alkoxy group is an alkyl group which is Single bond, singularly bonded to oxygen; thus . Denoted usually with apostrophe('). The range of alkoxy groups is vast, the simplest being methoxy (). An ethoxy group () is found in the ...
group at the β position to form intermediate vinyl zirconium complex. The E geometry selectivity is not cause by sterics but rather the reaction itself is not concerted. In a mechanistic study, they observed isomerization
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomer ...
, which suggest E geometry product is more favored than Z geometry. The difference of results between halogen exchange and E-vinyl ether reaction is that only when there is a presence of an oxonium intermediate, is isomerization
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomer ...
observed.
An interesting substitution reaction is vinyl boronic acid to vinyl iodide done by Brown's group. Depending on order of addition of iodide or base, vinyl borate
A borate is any of a range of boron oxyanions, anions containing boron and oxygen, such as orthoborate , metaborate , or tetraborate ; or any salt of such anions, such as sodium metaborate, and borax . The name also refers to esters of su ...
can yield different stereoisomers
In stereochemistry, stereoisomerism, or spatial isomerism, is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms (constitution), but differ in the three-dimensional orientations of their atoms i ...
of vinyl iodide (see scheme 2a). The Whiting group, however, noticed that Brown's method was not applicable to more sterically hindered boronic ester
A boronic acid is an organic compound related to boric acid () in which one of the three hydroxyl groups () is replaced by an alkyl or aryl group (represented by R in the general formula ). As a compound containing a carbon–boron bond, memb ...
s (no reaction). They proposed that the iodide source was not electropositive enough. So they decided to use ICl
ICL may refer to:
Companies and organizations
* Idaho Conservation League, environmental organisation in the United States
* Imperial College London, a UK university
* Indian Confederation of Labour
* Indian Cricket League
* Inorganic Chemistry ...
which is more polar than I2, in which, they observed similar results (see scheme 2b).
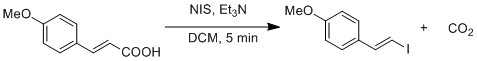
Radical substitution of carboxylic acid to iodide is demonstrated by a modified Hunsdiecker reaction
The Hunsdiecker reaction (also called the Borodin reaction or the Hunsdiecker–Borodin reaction) is a name reaction in organic chemistry whereby silver salts of carboxylic acids react with a halogen to produce an organic halide. It is an ex ...
. Homolytic cleavage of O-I bond generates CO2 and vinyl radical. Vinyl radical recombines with iodide radical to form vinyl iodide.

Iododesilylation
Iododesilylation is a substitution reaction ofsilyl
Silylation is the introduction of one or more (usually) substituted silyl groups (R3Si) to a molecule. Silylations are core methods for production of organosilicon chemistry. Silanization, while similar to silylation, usually refers to attachmen ...
group for iodide. The advantages of iododesilylation are that it avoids toxic tin reagent and intermediate vinyl silyl are stable, nontoxic and easily handled and stored. Vinyl silyl can be made from terminal alkyne or other methods.
The Kishi's group reported a mild preparation of vinyl iodide from vinyl silyl using NIS in mixture of acetonitrile
Acetonitrile, often abbreviated MeCN (methyl cyanide), is the chemical compound with the formula and structure . This colourless liquid is the simplest organic nitrile (hydrogen cyanide is a simpler nitrile, but the cyanide anion is not class ...
and chloroacetonitrile
Chloroacetonitrile is the organic compound with the formula ClCH2CN. A colorless liquid, it is derived from acetonitrile (CH3CN) by replacement of one H with Cl. In practice, it is produced by dehydration of chloroacetamide. The compound is an a ...
. They observed retention of olefin geometry in some vinyl silyl substrates while inversion in others. They reasoned that the R group's size had an effect on the geometry of the olefin. If the R group is small, the solvent acetonitrile
Acetonitrile, often abbreviated MeCN (methyl cyanide), is the chemical compound with the formula and structure . This colourless liquid is the simplest organic nitrile (hydrogen cyanide is a simpler nitrile, but the cyanide anion is not class ...
can participate in the reaction leading to inversion of the olefin's geometry. If the R group is big, the solvent is unable to participate, leading to retention of olefin's geometry
Zakarian's group then decided to run the reaction in HFIP, which gave high retention of olefin geometry. They reasoned that HFIP is a low nucleophilicity
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
solvent, unlike acetonitrile. In addition, they observed accelerated reaction rate because HFIP activate NIS by hydrogen bond
In chemistry, a hydrogen bond (H-bond) is a specific type of molecular interaction that exhibits partial covalent character and cannot be described as a purely electrostatic force. It occurs when a hydrogen (H) atom, Covalent bond, covalently b ...
ing.
Unfortunately, iododesilylation under those conditions (above) can potentially yield multiple byproducts in highly functionalized molecules with oxygen functional groups
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
. Vilarrasa and Costa's group hypothesized that radical reaction
A free-radical reaction is any chemical reaction involving free radicals. This reaction type is abundant in organic reactions. Two pioneering studies into free radical reactions have been the discovery of the triphenylmethyl radical by Moses Gomb ...
s producing HI and I2 help facilitate cleavage in alcohol's protecting group
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis.
In man ...
and may add into other alkene bonds. They experimented with the use of silver
Silver is a chemical element; it has Symbol (chemistry), symbol Ag () and atomic number 47. A soft, whitish-gray, lustrous transition metal, it exhibits the highest electrical conductivity, thermal conductivity, and reflectivity of any metal. ...
additives such as silver acetate
Silver acetate is a coordination compound with the empirical formula CH3CO2Ag (or AgC2H3O2). A photosensitive, white, crystalline solid, it is a useful reagent in the laboratory as a source of silver ions lacking an oxidizing anion.
Synthesis an ...
and silver carbonate
Silver carbonate is the chemical compound with the formula Ag2 C O3. This salt is yellow but typical samples are grayish due to the presence of elemental silver. It is poorly soluble in water, like most transition metal carbonates.
Preparation a ...
in which the silver can react with the excess iodide to form silver iodide
Silver iodide is an inorganic compound with the formula Ag I. The compound is a bright yellow solid, but samples almost always contain impurities of metallic silver that give a grey colouration. The silver contamination arises because some samp ...
. They achieved better conversions with these conditions.
Name reactions
Some famous vinyl iodide synthesis methods involve conversion ofaldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
or ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
to vinyl iodide. Barton's hydrazone iodination method involves addition of hydrazine
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly hazardous unless handled in solution as, for example, hydraz ...
s to aldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
or ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
to form hydrazone
Hydrazones are a class of organic compounds with the structure . They are related to ketones and aldehydes by the replacement of the oxygen =O with the = functional group. They are formed usually by the action of hydrazine on ketones or aldehydes. ...
. Then the hydrazone
Hydrazones are a class of organic compounds with the structure . They are related to ketones and aldehydes by the replacement of the oxygen =O with the = functional group. They are formed usually by the action of hydrazine on ketones or aldehydes. ...
is converted to vinyl iodide by addition of iodide and DBU DBU may refer to:
Universities
* Dallas Baptist University, Dallas, Texas, U.S.
* Desh Bhagat University, Mandi Gobindgarh, Punjab, India
* Duluth Business University, Duluth, Minnesota, U.S.
Other uses
* 1,8-Diazabicyclo .4.0ndec-7-ene, an org ...
. This method has been used in natural product synthesis of Taxol
Paclitaxel, sold under the brand name Taxol among others, is a chemotherapy medication used to treat ovarian cancer, esophageal cancer, breast cancer, lung cancer, Kaposi's sarcoma, cervical cancer, and pancreatic cancer. It is administered by ...
by Danishefsky and Cortistatin A by Shair.
Another method is the Takai olefination
Takai olefination in organic chemistry describes the organic reaction of an aldehyde with a diorganochromium compound to form an alkene. It is a name reaction, named for Kazuhiko Takai, who first reported it in 1986. In the original reaction, ...
which uses iodoform
Iodoform (also known as triiodomethane) is the organoiodine compound with the chemical formula . It is a pale yellow, crystalline, volatile substance, with a penetrating and distinctive odor (in older chemistry texts, the smell is sometimes refe ...
and chromium(II) chloride
Chromium(II) chloride describes inorganic compounds with the formula Cr Cl2(H2O)n. The anhydrous solid is white when pure, however commercial samples are often grey or green; it is hygroscopic and readily dissolves in water to give bright blue ai ...
to make vinyl iodide from aldehyde with high stereoselectivity
In chemistry, stereoselectivity is the property of a chemical reaction in which a single reactant forms an unequal mixture of stereoisomers during a non- stereospecific creation of a new stereocenter or during a non-stereospecific transformation ...
for E geometry. For high stereoselectivity
In chemistry, stereoselectivity is the property of a chemical reaction in which a single reactant forms an unequal mixture of stereoisomers during a non- stereospecific creation of a new stereocenter or during a non-stereospecific transformation ...
for Z geometry, Stork-Zhao olefination proceeds by Wittig Wittig is a surname, and may refer to:
* Burghardt Wittig (born 1947), German biochemist
* Curt Wittig, American chemist
* David Wittig (born 1955), American executive
* Edward Wittig (1879–1941), Polish sculptor
* Ferdinand Wittig (1851-1909), A ...
-like reaction. High yields and Z stereoselectivity
In chemistry, stereoselectivity is the property of a chemical reaction in which a single reactant forms an unequal mixture of stereoisomers during a non- stereospecific creation of a new stereocenter or during a non-stereospecific transformation ...
occurred at low temperature and at the presence of HMPA
Hexamethylphosphoramide, often abbreviated HMPA, is a phosphoramide (an amide of phosphoric acid) with the formula This colorless liquid is used as a solvent in organic synthesis.
Structure and reactivity
HMPA is the oxide of tris(dimethylamino ...
.
 Below is example of employing both Takai olefination and Stork-Zhao olefination in total synthesis of (+)-3-(E)- and (+)-3-(Z)-Pinnatifidenyne.
Below is example of employing both Takai olefination and Stork-Zhao olefination in total synthesis of (+)-3-(E)- and (+)-3-(Z)-Pinnatifidenyne.
Elimination method
Vinyl iodides are rarely by made an elimination reaction of vicinal diiodide because it tends to decompose to alkene and iodide. The Baker group have shown using decarboxylation, elimination can occur.See also
* List of functional groups *Group contribution method
A group-contribution method in chemistry is a technique to estimate and predict thermodynamic and other properties from molecular structures.
Introduction
In today's chemical processes hundreds of thousands of components are used. The Chemical A ...
References
{{Portal bar, Chemistry, Science, Technology Alkene derivatives Chemical synthesis Organoiodides