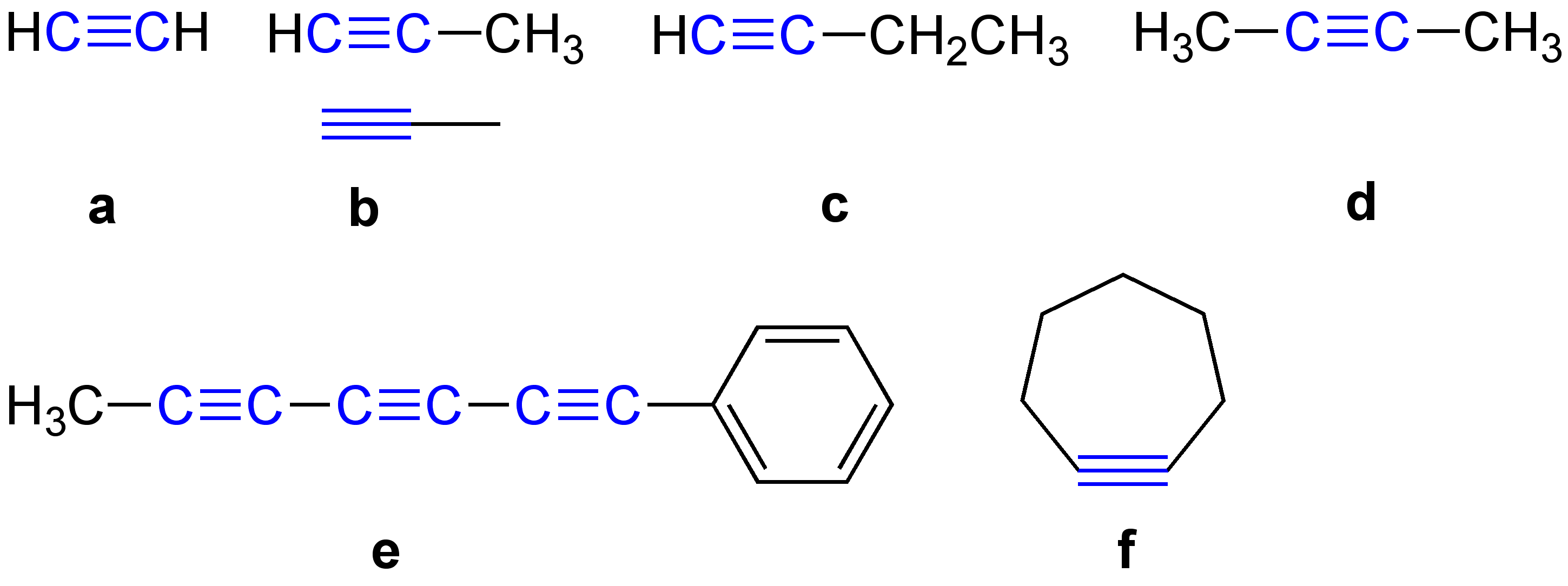
|
Alkyne
\ce \ce Acetylene \ce \ce \ce Propyne \ce \ce \ce \ce 1-Butyne In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula . Alkynes are traditionally known as acetylenes, although the name ''acetylene'' also refers specifically to , known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic. Structure and bonding In acetylene, the H–C≡C bond angles are 180°. By virtue of this bond angle, alkynes are rod-like. Correspondingly, cyclic alkynes are rare. Benzyne cannot be isolated. The C≡C bond distance of 118 picometers (for C2H2) is much shorter than the C=C distance in alkenes (132 pm, for C2H4) or the C–C bond in alkanes (153 pm). : The triple bond is very strong with a bond strength of 839 kJ/mol. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkyne General Formulae V
\ce \ce Acetylene \ce \ce \ce Propyne \ce \ce \ce \ce 1-Butyne In organic chemistry, an alkyne is an Saturated and unsaturated compounds, unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest Open-chain compound, acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula . Alkynes are traditionally known as acetylenes, although the name ''acetylene'' also refers specifically to , known formally as ethyne using IUPAC nomenclature of organic chemistry, IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic. Structure and bonding In acetylene, the H–C≡C bond angles are 180°. By virtue of this bond angle, alkynes are rod-like. Correspondingly, cyclic alkynes are rare. Benzyne cannot be isolated. The C≡C bond distance of 118 picometers (for C2H2) is much shorter than the C=C distance in alkenes (132 pm, for C2H4) or the C–C bond in alka ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and Hydrophobe, hydrophobic; their odor is usually faint, and may be similar to that of gasoline or Naphtha, lighter fluid. They occur in a diverse range of molecular structures and phases: they can be gases (such as methane and propane), liquids (such as hexane and benzene), low melting solids (such as paraffin wax and naphthalene) or polymers (such as polyethylene and polystyrene). In the fossil fuel industries, ''hydrocarbon'' refers to naturally occurring petroleum, natural gas and coal, or their hydrocarbon derivatives and purified forms. Combustion of hydrocarbons is the main source of the world's energy. Petroleum is the dominant raw-material source for organic commodity chemicals such as solvents and polymers. Most anthropogenic (human-generated) emissions of greenhouse gases are eithe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins. The International Union of Pure and Applied Chemistry (IUPAC) Preferred IUPAC name, recommends using the name "alkene" only for Open-chain compound, acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for Cyclic compound, cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula with ''n'' being a >1 natural number (which is two hydrogens less than the corresponding alkane). When ''n'' is four or more, isomers are possible, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1-Butyne
1-Butyne is an organic compound with the formula . It is a terminal alkyne. The compound is a common terminal alkyne substrate in diverse studies of catalysis. It is a colorless combustible gas. In 2017, was produced in the USA. 1-Butyne participates in reactions typical for terminal alkynes, such as alkyne metathesis, hydrogenation, condensation with formaldehyde. Based on its heat of combustion, it is slightly more stable than its isomer 2-butyne. The combustion of 1-Butyne produces propargyl radicals, a pre-cursor to soot and polycyclic aromatic hydrocarbons, as the propargyl radicals can form basic aromatic rings, making butyne's fuel usage a concern for emissions. 1-Butyne is in unsaturated C4 petroleum cuts, and has to be separated out in industrial hydrorefining to make 1-butene, which is used to make low density polyethylene and polybutene. Distillation is impractical due to similar boiling points, so 1-butyne is removed by catalytic hydrogenation. Usually the cata ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetylene
Acetylene (Chemical nomenclature, systematic name: ethyne) is a chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in its pure form and thus is usually handled as a solution. Pure acetylene is odorless, but commercial grades usually have a marked odor due to impurities such as divinyl sulfide and phosphine.Compressed Gas Association (1995Material Safety and Data Sheet – Acetylene As an alkyne, acetylene is Saturated and unsaturated compounds, unsaturated because its two carbon atoms are Chemical bond, bonded together in a triple bond. The carbon–carbon triple bond places all four atoms in the same straight line, with CCH bond angles of 180°. The triple bond in acetylene results in a high energy content that is released when acetylene is burned. Discovery Acetylene was discovered in 1836 by Edmund Davy, who identified it as a "new carburet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethyne
Acetylene (systematic name: ethyne) is a chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in its pure form and thus is usually handled as a solution. Pure acetylene is odorless, but commercial grades usually have a marked odor due to impurities such as divinyl sulfide and phosphine.Compressed Gas Association (1995Material Safety and Data Sheet – Acetylene As an alkyne, acetylene is unsaturated because its two carbon atoms are bonded together in a triple bond. The carbon–carbon triple bond places all four atoms in the same straight line, with CCH bond angles of 180°. The triple bond in acetylene results in a high energy content that is released when acetylene is burned. Discovery Acetylene was discovered in 1836 by Edmund Davy, who identified it as a "new carburet of hydrogen". It was an accidental discovery while attempting to isolate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diphenylacetylene
Diphenylacetylene is the chemical compound C6H5C≡CC6H5. The molecule consists of two phenyl groups attached to a C2 unit. A colorless solid, it is used as a building block in organic synthesis and as a Transition metal alkyne complex, ligand in organometallic chemistry. Preparation and structure In one preparation for this compound, benzil is condensed with hydrazine to give the bis(hydrazone), which is oxidized with mercury(II) oxide. Alternatively stilbene is brominated, and the resulting dibromodiphenylethane is subjected to dehydrohalogenation. Yet another method involves the coupling of iodobenzene and the copper salt of phenylacetylene in the Castro-Stephens coupling. The related Sonogashira coupling involves the coupling of iodobenzene and phenylacetylene. Diphenylacetylene is a planar molecule. The central C≡C distance is 119.8 picometers. Derivatives 1,2,3,4-Tetraphenylbutadiene is prepared by reductive coupling of diphenylacetylene using lithium metal followed by h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes Physical property, physical and Chemical property, chemical properties, and evaluation of Reactivity (chemistry), chemical reactivity to understand their behavior. The study of organic reactions includes the organic synthesis, chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical (in silico) study. The range of chemicals studied chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triple Bond
A triple bond in chemistry is a chemical bond between two atoms involving six Electron pair bond, bonding electrons instead of the usual two in a covalent bond, covalent single bond. Triple bonds are stronger than the equivalent covalent bond, single bonds or double bond, double bonds, with a bond order of three. The most common triple bond is in a nitrogen N2 molecule; the second most common is that between two carbon atoms, which can be found in alkynes. Other functional groups containing a triple bond are cyanides and isocyanides. Some diatomic molecules, such as diphosphorus and carbon monoxide, are also triple bonded. In skeletal formula, skeletal formulae the triple bond is drawn as three parallel lines (≡) between the two connected atoms. Bonding Triple bonding can be explained in terms of orbital hybridization. In the case of acetylene, each carbon atom has two sp orbital, sp-orbitals and two p-orbitals. The two sp-orbitals are linear, with 180° bond angles, and occupy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IUPAC Nomenclature Of Organic Chemistry
In chemical nomenclature, the IUPAC nomenclature of organic chemistry is a method of naming organic chemical compounds as recommended by the International Union of Pure and Applied Chemistry (IUPAC). It is published in the '' Nomenclature of Organic Chemistry'' (informally called thBlue Book. Ideally, every possible organic compound should have a name from which an unambiguous structural formula can be created. There is also an IUPAC nomenclature of inorganic chemistry. To avoid long and tedious names in normal communication, the official IUPAC naming recommendations are not always followed in practice, except when it is necessary to give an unambiguous and absolute definition to a compound. IUPAC names can sometimes be simpler than older names, as with ethanol, instead of ethyl alcohol. For relatively simple molecules they can be more easily understood than non-systematic names, which must be learnt or looked over. However, the common or trivial name is often substantially ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propyne
Propyne (methylacetylene) is an alkyne with the chemical formula . It is a component of MAPD gas—along with its isomer propadiene (allene), which was commonly used in gas welding. Unlike acetylene, propyne can be safely condensed.Peter Pässler, Werner Hefner, Klaus Buckl, Helmut Meinass, Andreas Meiswinkel, Hans-Jürgen Wernicke, Günter Ebersberg, Richard Müller, Jürgen Bässler, Hartmut Behringer, Dieter Mayer, "Acetylene" in Ullmann's Encyclopedia of Industrial Chemistry Wiley-VCH, Weinheim 2007 (). Production and equilibrium with propadiene Propyne exists in equilibrium with propadiene, the mixture of propyne and propadiene being called MAPD: : The coefficient of equilibrium ''K''eq is 0.22 at 270 °C or 0.1 at 5 °C. MAPD is produced as a side product, often an undesirable one, by cracking propane to produce propene, an important feedstock in the chemical industry. MAPD interferes with the catalytic polymerization of propene. Laboratory methods Propyn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orbital Hybridisation
In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new ''hybrid orbitals'' (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to form chemical bonds in valence bond theory. For example, in a carbon atom which forms four single bonds, the valence-shell s orbital combines with three valence-shell p orbitals to form four equivalent sp3 mixtures in a tetrahedral arrangement around the carbon to bond to four different atoms. Hybrid orbitals are useful in the explanation of molecular geometry and atomic bonding properties and are symmetrically disposed in space. Usually hybrid orbitals are formed by mixing atomic orbitals of comparable energies. History and uses Chemist Linus Pauling first developed the hybridisation theory in 1931 to explain the structure of simple molecules such as methane (CH4) using atomic orbitals. Pauling pointed out that a carbon atom forms four ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |