|
Chromium(II) Chloride
Chromium(II) chloride describes inorganic compounds with the formula Cr Cl2(H2O)n. The anhydrous solid is white when pure, however commercial samples are often grey or green; it is hygroscopic and readily dissolves in water to give bright blue air-sensitive solutions of the tetrahydrate Cr(H2O)4Cl2. Chromium(II) chloride has no commercial uses but is used on a laboratory-scale for the synthesis of other chromium complexes. Synthesis CrCl2 is produced by reducing chromium(III) chloride either with hydrogen at 500 °C: :2CrCl3 + H2 → 2CrCl2 + 2HCl or by electrolysis. On the laboratory scale, LiAlH4, zinc, and related reductants produce chromous chloride from chromium(III) precursors: :4 CrCl3 + LiAlH4 → 4 CrCl2 + LiCl + AlCl3 + 2 H2 :2 CrCl3 + Zn → 2 CrCl2 + ZnCl2 CrCl2 can also be prepared by treating a solution of chromium(II) acetate with hydrogen chloride: :Cr2(OAc)4 + 4 HCl → 2 CrCl2 + 4 AcOH Treatment of chromium powder with concentrated hydrochloric acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the pseudoelement symbol for ethyl group, ethyl. Ethanol is a Volatility (chemistry), volatile, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. As a psychoactive depressant, it is the active ingredient in alcoholic beverages, and the second most consumed drug globally behind caffeine. Ethanol is naturally produced by the fermentation process of sugars by yeasts or via petrochemical processes such as ethylene hydration. Historically it was used as a general anesthetic, and has modern medical applications as an antiseptic, disinfectant, solvent for some medications, and antidote for methanol poisoning and ethylene glycol poisoning. It is used as a chemical solvent and in the Chemical synthesis, synthesis of orga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorine
Chlorine is a chemical element; it has Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidizing agent, oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity on the revised Electronegativity#Pauling electronegativity, Pauling scale, behind only oxygen and fluorine. Chlorine played an important role in the experiments conducted by medieval Alchemy, alchemists, which commonly involved the heating of chloride Salt (chemistry), salts like ammonium chloride (sal ammoniac) and sodium chloride (common salt), producing various chemical substances containing chlorine such as hydrogen chloride, mercury(II) chloride (corrosive sublimate), and . However, the nature of fre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
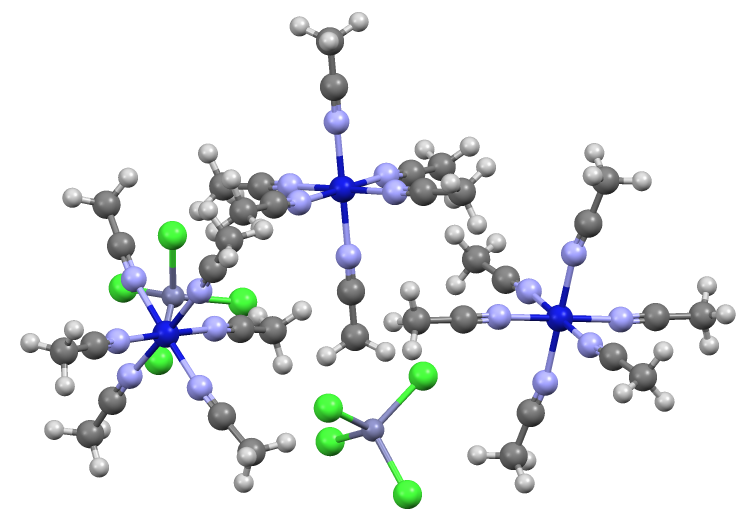
Octahedral Molecular Geometry
In chemistry, octahedral molecular geometry, also called square bipyramidal, describes the shape of compounds with six atoms or groups of atoms or ligands symmetrically arranged around a central atom, defining the vertices of an octahedron. The octahedron has eight faces, hence the prefix '' octa''. The octahedron is one of the Platonic solids, although octahedral molecules typically have an atom in their centre and no bonds between the ligand atoms. A perfect octahedron belongs to the point group Oh. Examples of octahedral compounds are sulfur hexafluoride SF6 and molybdenum hexacarbonyl Mo(CO)6. The term "octahedral" is used somewhat loosely by chemists, focusing on the geometry of the bonds to the central atom and not considering differences among the ligands themselves. For example, , which is not octahedral in the mathematical sense due to the orientation of the bonds, is referred to as octahedral. The concept of octahedral coordination geometry was developed by Alfred ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Chloride
Calcium chloride is an inorganic compound, a Salt (chemistry), salt with the chemical formula . It is a white crystalline solid at room temperature, and it is highly soluble in water. It can be created by neutralising hydrochloric acid with calcium hydroxide. Calcium chloride is commonly encountered as a Water of crystallization, hydrated solid with generic formula , where ''n'' = 0, 1, 2, 4, and 6. These compounds are mainly used for de-icing and dust control. Because the anhydrous salt is Hygroscopic, hygroscopic and deliquescent, it is used as a desiccant.Robert Kemp, Suzanne E. Keegan "Calcium Chloride" in Ullmann's Encyclopedia of Industrial Chemistry 2000, Wiley-VCH, Weinheim. History Calcium chloride was apparently discovered in the 15th century but wasn't studied properly until the 18th century. It was historically called "fixed Salammoniac, sal ammoniac" () because it was synthesized during the distillation of ammonium chloride with lime and was nonvolatile (while ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Space Group
In mathematics, physics and chemistry, a space group is the symmetry group of a repeating pattern in space, usually in three dimensions. The elements of a space group (its symmetry operations) are the rigid transformations of the pattern that leave it unchanged. In three dimensions, space groups are classified into 219 distinct types, or 230 types if chiral copies are considered distinct. Space groups are discrete cocompact groups of isometries of an oriented Euclidean space in any number of dimensions. In dimensions other than 3, they are sometimes called Bieberbach groups. In crystallography, space groups are also called the crystallographic or Fedorov groups, and represent a description of the symmetry of the crystal. A definitive source regarding 3-dimensional space groups is the ''International Tables for Crystallography'' . History Space groups in 2 dimensions are the 17 wallpaper groups which have been known for several centuries, though the proof that the list ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetonitrile Complex
Transition metal nitrile complexes are coordination compounds containing nitrile ligands. Because nitriles are weakly basic, the nitrile ligands in these complexes are often labile. Ligand properties According to the Covalent bond classification method, nitriles are classified as L ligands, i.e., charge-neutral Lewis bases. With respect to HSAB theory, they are classified as soft. Typical nitrile ligands are acetonitrile, propionitrile, and benzonitrile. The structures of u(NH3)5(NCPh)sup>n+ have been determined for the 2+ and 3+ oxidation states. Upon oxidation the Ru-NH3 distances contract and the Ru-NCPh distances elongate, consistent with amines serving as pure-sigma donor ligands and nitriles functioning as pi-acceptors. Synthesis and reactions Acetonitrile, propionitrile and benzonitrile are also popular solvents. Because nitrile solvents have high dielectric constants, cationic complexes containing a nitrile ligand are often soluble in a solution of that nitrile. Some ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrochloric Acid
Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungency, pungent smell. It is classified as a acid strength, strong acid. It is a component of the gastric acid in the digestive systems of most animal species, including humans. Hydrochloric acid is an important laboratory reagent and industrial chemical. Etymology Because it was produced from halite, rock salt according to the methods of Johann Rudolph Glauber, hydrochloric acid was historically called by European alchemists ''spirits of salt'' or ''acidum salis'' (salt acid). Both names are still used, especially in other languages, such as , , , , , , , , , , (''ensan''), zh, 盐酸 (''yánsuān''), and (''yeomsan''). Gaseous HCl was called ''marine acid air''. The name ''muriatic acid'' has the same origin (''muriatic'' means "pertaining to brine or salt", hence ''muriate'' means hydrochloride), and this ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Chloride
The Chemical compound, compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colorless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hydrogen chloride gas and hydrochloric acid are important in technology and industry. Hydrochloric acid, the aqueous solution of hydrogen chloride, is also commonly given the formula HCl. Reactions Hydrogen chloride is a diatomic molecule, consisting of a hydrogen atom H and a chlorine atom Cl connected by a Polar-covalent bond, polar covalent bond. The chlorine atom is much more Electronegativity, electronegative than the hydrogen atom, which makes this bond polar. Consequently, the molecule has a large Molecular dipole moment, dipole moment with a negative partial charge (δ−) at the chlorine atom and a positive partial charge (δ+) at the hydrogen atom. In part because of its high polarity, HCl is very soluble in water (and in other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromium(II) Acetate
Chromium(II) acetate hydrate, also known as chromous acetate, is the coordination compound with the chemical formula, formula Cr2(CH3CO2)4(H2O)2. This formula is commonly abbreviated Cr2(OAc)4(H2O)2. This red-coloured compound features a quadruple bond. It exists as the dihydrate and the anhydrous forms. Both are diamagnetic. Cr2(OAc)4(H2O)2 is a reddish diamagnetic powder, although diamond-shaped tabular crystals can be grown. Consistent with the fact that it is nonionic, Cr2(OAc)4(H2O)2 exhibits poor solubility in water and methanol. Structure The Cr2(OAc)4(H2O)2 molecule contains two atoms of chromium, two ligand, ligated molecules of water, and four acetate bridging ligands. The coordination environment around each chromium atom consists of four oxygen atoms (one from each acetate ligand) in a square, one water molecule (in an axial position), and the other chromium atom (opposite the water molecule), giving each chromium centre an Octahedral molecular geometry, octahedral ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc
Zinc is a chemical element; it has symbol Zn and atomic number 30. It is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic table. In some respects, zinc is chemically similar to magnesium: both elements exhibit only one normal oxidation state (+2), and the Zn2+ and Mg2+ ions are of similar size. Zinc is the 24th most abundant element in Earth's crust and has five stable isotopes. The most common zinc ore is sphalerite (zinc blende), a zinc sulfide mineral. The largest workable lodes are in Australia, Asia, and the United States. Zinc is refined by froth flotation of the ore, roasting, and final extraction using electricity ( electrowinning). Zinc is an essential trace element for humans, animals, plants and for microorganisms and is necessary for prenatal and postnatal development. It is the second most abundant trace metal in humans after iron, an import ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Aluminium Hydride
Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula or . It is a white solid, discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters, carboxylic acids, and amides. The solid is dangerously reactive toward water, releasing gaseous hydrogen (H2). Some related derivatives have been discussed for hydrogen storage. Properties, structure, preparation LAH is a colourless solid but commercial samples are usually gray due to contamination. This material can be purified by recrystallization from diethyl ether. Large-scale purifications employ a Soxhlet extractor. Commonly, the impure gray material is used in synthesis, since the impurities are innocuous and can be easily separated from the organic products. The pure powdered material is pyrophoric, but not its large crystals. Some commercial materials contain mineral oil to inhibit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |