Acetonitrile Complex on:
[Wikipedia]
[Google]
[Amazon]
Transition metal nitrile complexes are
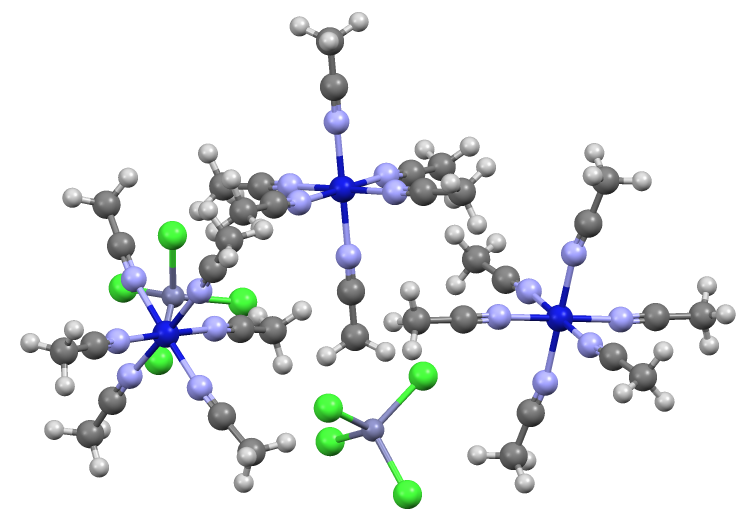 For the synthesis of some acetonitrile complexes, the nitrile serves as a reductant. This method is illustrated by the conversion of
For the synthesis of some acetonitrile complexes, the nitrile serves as a reductant. This method is illustrated by the conversion of

coordination compound
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of chemical bond, bound molecules or ions, that are in turn known as ' ...
s containing nitrile
In organic chemistry, a nitrile is any organic compound that has a functional group. The name of the compound is composed of a base, which includes the carbon of the , suffixed with "nitrile", so for example is called " propionitrile" (or pr ...
ligands. Because nitriles are weakly basic, the nitrile ligands in these complexes are often labile
Lability refers to the degree that something is likely to undergo change. It is the opposite ( antonym) of stability.
Biochemistry
In reference to biochemistry, this is an important concept as far as kinetics is concerned in metalloprotein ...
.
Ligand properties
According to theCovalent bond classification method The covalent bond classification (CBC) method, also referred to as LXZ notation, is a way of describing covalent compounds such as organometallic complexes in a way that is not prone to limitations resulting from the definition of oxidation state. ...
, nitriles are classified as L ligands, i.e., charge-neutral Lewis bases. With respect to HSAB theory
HSAB is an acronym for "hard and soft (Lewis) acids and bases". HSAB is widely used in chemistry for explaining the stability of compounds, reaction mechanisms and pathways. It assigns the terms 'hard' or 'soft', and 'acid' or 'base' to chemical ...
, they are classified as soft.
Typical nitrile ligands are acetonitrile
Acetonitrile, often abbreviated MeCN (methyl cyanide), is the chemical compound with the formula and structure . This colourless liquid is the simplest organic nitrile (hydrogen cyanide is a simpler nitrile, but the cyanide anion is not class ...
, propionitrile
Propionitrile, also known as ethyl cyanide and propanenitrile, is an organic compound with the formula CH3CH2CN. It is a simple aliphatic nitrile. The compound is a colourless, water-soluble liquid. It is used as a solvent and a precursor to oth ...
, and benzonitrile
Benzonitrile is the chemical compound with the formula , abbreviated PhCN. This aromatic organic compound is a colorless liquid with a cherry or almond like odour. It is mainly used industrially to produce the melamine resin precursor benzoguanam ...
. The structures of u(NH3)5(NCPh)sup>n+ have been determined for the 2+ and 3+ oxidation states. Upon oxidation the Ru-NH3 distances contract and the Ru-NCPh distances elongate, consistent with amines serving as pure-sigma donor ligands and nitriles functioning as pi-acceptors.
Synthesis and reactions
Acetonitrile, propionitrile and benzonitrile are also popular solvents. Because nitrilesolvent
A solvent (from the Latin language, Latin ''wikt:solvo#Latin, solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a Solution (chemistry), solution. A solvent is usually a liquid but can also be a solid, a gas ...
s have high dielectric constant
The relative permittivity (in older texts, dielectric constant) is the permittivity of a material expressed as a ratio with the electric permittivity of a vacuum. A dielectric is an insulating material, and the dielectric constant of an insul ...
s, cationic complexes containing a nitrile ligand are often soluble in a solution of that nitrile.
Some complexes can be prepared by dissolving an anhydrous metal salt in the nitrile. In other cases, a suspension of the metal is oxidized with a solution of NOBF4 in the nitrile:
:Ni + 6 MeCN + 2 NOBF4 → i(MeCN)6BF4)2 + 2 NO
Heteroleptic complexes of molybdenum and tungsten can by synthesized from their respective hexacarbonyl complexes.
:M(CO)6 + 4 MeCN + 2 NOBF4 → (NO)2(MeCN)4BF4)2
 For the synthesis of some acetonitrile complexes, the nitrile serves as a reductant. This method is illustrated by the conversion of
For the synthesis of some acetonitrile complexes, the nitrile serves as a reductant. This method is illustrated by the conversion of molybdenum pentachloride
Molybdenum(V) chloride is the inorganic compound with the empirical formula . This dark volatile solid is used in research to prepare other molybdenum compounds. It is moisture-sensitive and soluble in chlorinated solvents.
Structure
Usually cal ...
to the molybdenum(IV) complex:
: 2 MoCl5 + 5 CH3CN → 2 MoCl4(CH3CN)2 + ClCH2CN + HCl
Reactions
Transition metal nitrile complexes are usually employed because the nitrile ligand is labile and relatively chemically inert. Cationic nitrile complexes are however susceptible to nucleophilic attack at carbon. Consequently some nitrile complexes catalyze the hydrolysis of nitriles to give the amides. Fe- and Co-nitrile complexes are intermediates innitrile hydratase
Nitrile hydratases (NHases; ) are mononuclear iron or non-corrinoid cobalt enzymes that catalyse the hydration of diverse nitriles to their corresponding amides:
: R-C≡N + →
Metal cofactor
Nitrile hydratases use Fe(III) or Co(III) at thei ...
enzymes. N-coordination activates the sp-hybridized carbon center toward attack by nucleophiles, including water. Thus coordination of the nitrile to a cationic metal center is the basis for the catalytic hydration:
:M-NCR + H2O → M-O=C(NH2)R
:M-O=C(NH2)R + NCR → O=C(NH2)R + M-NCR
Nitrile ligands in electron-rich
Electron-rich is jargon that is used in multiple related meanings with either or both kinetic and thermodynamic implications:
* with regards to electron-transfer, electron-rich species have low ionization energy and/or are reducing agents. Tetr ...
complexes are susceptible to oxidation, e.g. by iodosylbenzene
Iodosobenzene or iodosylbenzene is an organoiodine compound with the empirical formula . This colourless solid compound is used as an oxo transfer reagent in research laboratories examining organic and coordination chemistry.
Preparation and stru ...
. Nitriles undergo coupling with alkenes, also involving electron-rich complexes.
Examples
(NCMe)6sup>n+
* Hexakis(acetonitrile)vanadium(II) tetrachlorozincate ( (MeCN)6ZnCl4)), green * Hexakis(acetonitrile)chromium(II) bis(tetraphenylborate) ( r(MeCN)6B(C6H5)4)2, green * Hexakis(acetonitrile)chromium(III) tetrafluoroborate ( r(MeCN)6BF4)3), white * Hexakis(acetonitrile)iron(II) bis(tetrakis(pentafluorophenyl)borate) ( e(MeCN)6B(C6F5)4)2, orange * Hexakis(acetonitrile)cobalt(II) bis(tetrakis(pentafluorophenyl)borate) ( o(MeCN)6B(C6F5)4)2, purple * Hexakis(acetonitrile)nickel(II) tetrafluoroborate ( i(MeCN)6BF4)2), blue * Hexakis(acetonitrile)copper(II) bis(tetrakis(pentafluorophenyl)borate) ( u(MeCN)6B(C6F5)4)2, pale blue-green solid * Hexakis(acetonitrile)ruthenium(II) tetrafluoroborate ( u(MeCN)6BF4)2), white, dRu-N = 202 pm. * Hexakis(acetonitrile)rhodium(III) tetrafluoroborate ( h(MeCN)6BF4)3), a yellow solid. * Hexakis(acetonitrile)rhenium(II) tetrafluoroborate ( e(MeCN)6BF4)2), a yellow solid. * Hexakis(acetonitrile)rhenium(III) tetrafluoroborate ( e(MeCN)6BF4)3), a brown solid.(NCMe)4sup>n+
* r(MeCN)4BF4)2, blue * u(MeCN)4F6, colorless * d(MeCN)4BF4)2, yellow(NCMe)4 or 5sub>2n+
* o2(MeCN)8/10BF4)4 blue d(Mo-Mo) = 218, d(Mo-N)axial = 260, d(Mo-N)equat = 214 pm * c2(MeCN)10BF4)4 * e2(MeCN)10B(C6H3(CF3)2)4]2, blue; d(Re-Re) = 226, d(Re-N)axial = 240, d(Re-N)equat = 205 pm * h2(MeCN)10BF4)4, orange; d(Rh-Rh) = 261, d(Re-N)axial = 219, d(Re-N)equat = 198 pm(NCMe)2sup>+
* g(MeCN)2(C6H3(CF3)2)4 * u(MeCN)2bF6Mixed ligand examples
*Bis(benzonitrile)palladium dichloride
Bis(benzonitrile)palladium dichloride is the coordination complex with the formula PdCl2(NCC6H5)2. It is the adduct of two benzonitrile (PhCN) ligands with palladium(II) chloride. It is a yellow-brown solid that is soluble in organic solvents. ...
(PdCl2(PhCN)2), an orange solid that serves as a source of "PdCl2"
* Tricarbonyltris(propionitrile)molybdenum(0) (Mo(CO)3(C2H5CN)3), a source of "Mo(CO)3". Related Cr and W complexes are known.
Complexes of η2-nitrile ligands
Cases are known where nitriles function as η2-ligands. This bonding mode is more common for complexes of low-valence metals, such as Ni(0). Complexes of η2-nitriles are expected to form as transient intermediates in certain metal-catalyzed reactions of nitriles, such as theHoesch reaction
The Hoesch reaction or Houben–Hoesch reaction is an organic reaction in which a nitrile reacts with an arene compound to form an aryl ketone. The reaction is a type of Friedel–Crafts acylation with hydrogen chloride and a Lewis acid catalyst.
...
and the hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated ...
of nitriles.
In some cases, η2-nitrile ligands are intermediates that preceded oxidative addition
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre. Oxidat ...
.

See also
*Cyanometalate
Cyanometallates or cyanometalates are a class of coordination compounds, most often consisting only of cyanide ligands. Most are anions. Cyanide is a highly basic and small ligand, hence it readily saturates the coordination sphere of metal ions. ...
– coordination compounds containing cyanide ligands (coordinating via C).
References