|
Silyl
Silylation is the introduction of one or more (usually) substituted silyl groups (R3Si) to a molecule. Silylations are core methods for production of organosilicon chemistry. Silanization, while similar to silylation, usually refers to attachment of silyl groups to solids. Silyl groups are commonly used for: alcohol protection, enolate trapping, gas chromatography, electron-impact mass spectrometry (EI-MS), and coordinating with metal complexes. Protection Chemistry Protection Silylation is often used to protect alcohols, as well as amines, carboxylic acids, and terminal alkynes. The products after silylation, namely silyl ethers and silyl amines, are resilient toward basic conditions. Protection is typically done by reacting the functional group with a silyl halide by an SN2 reaction mechanism, typically in the presence of base. The protection mechanism begins with the base deprotonating the alcohol group. Next, the deprotonated alcohol group attacks the silyl atom of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
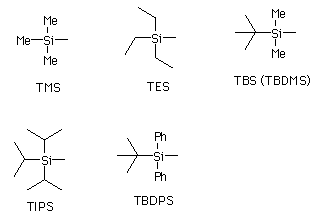
Silyl Ether
Silyl ethers are a group of chemical compounds which contain a silicon atom covalently bonded to an alkoxy group. The general structure is R1R2R3Si−O−R4 where R4 is an alkyl group or an aryl group. Silyl ethers are usually used as protecting groups for alcohols in organic synthesis. Since R1R2R3 can be combinations of differing groups which can be varied in order to provide a number of silyl ethers, this group of chemical compounds provides a wide spectrum of selectivity for protecting group chemistry. Common silyl ethers are: trimethylsilyl (TMS), ''tert''-butyldiphenylsilyl (TBDPS), ''tert''-butyldimethylsilyl (TBS/TBDMS) and triisopropylsilyl (TIPS). They are particularly useful because they can be installed and removed very selectively under mild conditions. Common silyl ethers Formation Commonly silylation of alcohols requires a silyl chloride and an amine base. One reliable and rapid procedure is the Corey protocol in which the alcohol is reacted with a silyl c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
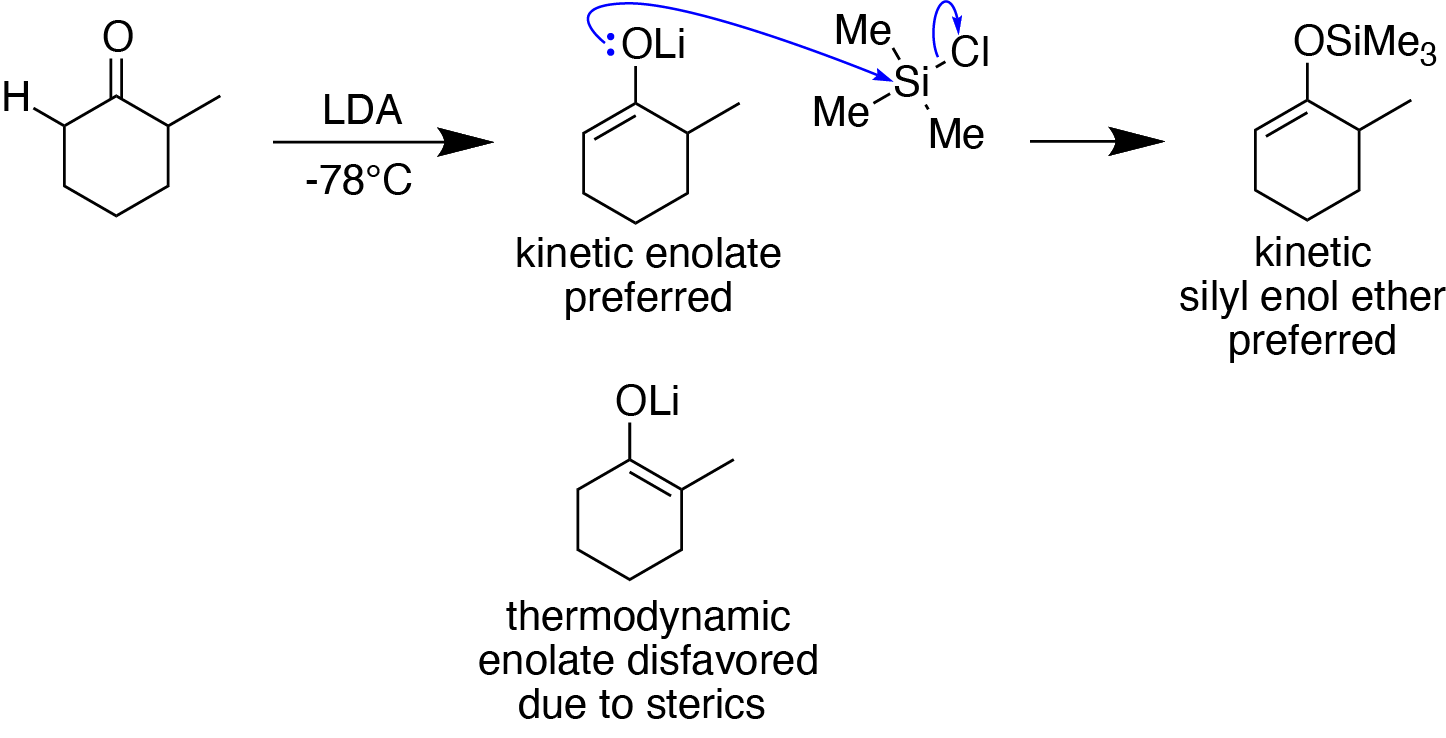
Silyl Enol Ether
In organosilicon chemistry, silyl enol ethers are a class of organic compounds that share the common functional group , composed of an enolate () bonded to a silane () through its oxygen end and an ethene group () as its carbon end. They are important intermediates in organic synthesis. Synthesis Silyl enol ethers are generally prepared by reacting an enolizable carbonyl compound with a silyl electrophile and a base, or just reacting an enolate with a silyl electrophile.Clayden, J., Greeves, N., & Warren, S. (2012). Silyl enol ethers. In ''Organic chemistry'' (Second ed., pp. 466-467). Oxford University Press. Since silyl electrophiles are hard and silicon-oxygen bonds are very strong, the oxygen (of the carbonyl compound or enolate) acts as the nucleophile to form a Si-O single bond. The most commonly used silyl electrophile is trimethylsilyl chloride. To increase the rate of reaction, trimethylsilyl triflate may also be used in the place of trimethylsilyl chloride as a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Silyl
Silylation is the introduction of one or more (usually) substituted silyl groups (R3Si) to a molecule. Silylations are core methods for production of organosilicon chemistry. Silanization, while similar to silylation, usually refers to attachment of silyl groups to solids. Silyl groups are commonly used for: alcohol protection, enolate trapping, gas chromatography, electron-impact mass spectrometry (EI-MS), and coordinating with metal complexes. Protection Chemistry Protection Silylation is often used to protect alcohols, as well as amines, carboxylic acids, and terminal alkynes. The products after silylation, namely silyl ethers and silyl amines, are resilient toward basic conditions. Protection is typically done by reacting the functional group with a silyl halide by an SN2 reaction mechanism, typically in the presence of base. The protection mechanism begins with the base deprotonating the alcohol group. Next, the deprotonated alcohol group attacks the silyl atom of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Silylation Protection Scheme
Silylation is the introduction of one or more (usually) substituted silyl groups (R3Si) to a molecule. Silylations are core methods for production of organosilicon chemistry. Silanization, while similar to silylation, usually refers to attachment of silyl groups to solids. Silyl groups are commonly used for: alcohol protection, enolate trapping, gas chromatography, electron-impact mass spectrometry (EI-MS), and coordinating with metal complexes. Protection Chemistry Protection Silylation is often used to protect alcohols, as well as amines, carboxylic acids, and terminal alkynes. The products after silylation, namely silyl ethers and silyl amines, are resilient toward basic conditions. Protection is typically done by reacting the functional group with a silyl halide by an SN2 reaction mechanism, typically in the presence of base. The protection mechanism begins with the base deprotonating the alcohol group. Next, the deprotonated alcohol group attacks the silyl atom of the s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Trimethylsilyl Chloride
Trimethylsilyl chloride, also known as chlorotrimethylsilane is an organosilicon compound ( silyl halide), with the formula , often abbreviated or TMSCl. It is a colourless volatile liquid that is stable in the absence of water. It is widely used in organic chemistry. Preparation TMSCl is prepared on a large scale by the '' direct process'', the reaction of methyl chloride with a silicon-copper alloy. The principal target of this process is dimethyldichlorosilane, but substantial amounts of the trimethyl and monomethyl products are also obtained. The relevant reactions are (Me = methyl, ): x\ \ce \longrightarrow \begin \ce, \\ pt \ce, \\ pt \ce,\\ pt \text \end Typically about 2–4% of the product stream is the monochloride, which forms an azeotrope with . Reactions and uses TMSCl is reactive toward nucleophiles, resulting in the replacement of the chloride. In a characteristic reaction of TMSCl, the nucleophile is water, resulting in hydrolysis to give the hexa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Organosilicon Compound
Organosilicon chemistry is the study of organometallic compounds containing carbon–silicon bonds, to which they are called organosilicon compounds. Most organosilicon compounds are similar to the ordinary organic compounds, being colourless, flammable, hydrophobic, and stable to air. Silicon carbide is an ''inorganic'' compound. History In 1863, Charles Friedel and James Crafts made the first organochlorosilane compound. The same year, they also described a "polysilicic acid ether" in the preparation of ethyl- and methyl-o-silicic acid. Extensive research in the field of organosilicon compounds was pioneered in the beginning of 20th century by Frederic S. Kipping. He also had coined the term "silicone" (resembling ''ketones'', though this is erroneous) in relation to these materials in 1904. In recognition of Kipping's achievements, the Dow Chemical Company had established an award in the 1960s that is given for significant contributions to the field of silicon chemistry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Organosilicon Chemistry
Organosilicon chemistry is the study of organometallic compounds containing carbon–silicon bonds, to which they are called organosilicon compounds. Most organosilicon compounds are similar to the ordinary organic compounds, being colourless, flammable, hydrophobic, and stable to air. Silicon carbide is an ''inorganic'' compound. History In 1863, Charles Friedel and James Crafts made the first organochlorosilane compound. The same year, they also described a "polysilicic acid ether" in the preparation of ethyl- and methyl-o-silicic acid. Extensive research in the field of organosilicon compounds was pioneered in the beginning of 20th century by Frederic S. Kipping. He also had coined the term "silicone" (resembling ''ketones'', though this is erroneous) in relation to these materials in 1904. In recognition of Kipping's achievements, the Dow Chemical Company had established an award in the 1960s that is given for significant contributions to the field of silicon chemistry. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Hydrosilylation
Hydrosilylation, also called catalytic hydrosilation, describes the addition of Si-H bonds across unsaturated bonds."Hydrosilylation A Comprehensive Review on Recent Advances" B. Marciniec (ed.), Advances in Silicon Science, Springer Science, 2009. Ordinarily the reaction is conducted catalytically and usually the substrates are unsaturated organic compounds. Alkenes and alkynes give alkyl and vinyl silanes; aldehydes and ketones give silyl ethers, while esters provide alkyl silyl mixed acetals. Hydrosilylation has been called the "most important application of platinum in homogeneous catalysis." Scope and mechanism Hydrosilylation of alkenes represents a commercially important method for preparing organosilicon compounds. The process is mechanistically similar to the hydrogenation of alkenes. In fact, similar catalysts are sometimes employed for the two catalytic processes. The prevalent mechanism, called the Chalk-Harrod mechanism, assumes an intermediate metal complex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Bis(trimethylsilyl)acetamide
Bis(trimethylsilyl)acetamide (BSA) is an organosilicon compound with the formula (Me = CH3). It is a colorless liquid that is soluble in diverse organic solvents, but reacts rapidly with moisture and solvents containing OH and NH groups. It is used in analytical chemistry to increase the volatility of analytes, e.g., for gas chromatography. It is also used to introduce the trimethylsilyl protecting group in organic synthesis.Harry Heaney, Jian Cui, “N,O-Bis(trimethylsilyl)acetamide” Encyclopedia of Reagents for Organic Synthesis Copyright © 2007 John Wiley & Sons. . A related reagent is N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA). Synthesis and reactions BSA is prepared by treating acetamide with trimethylsilyl chloride Trimethylsilyl chloride, also known as chlorotrimethylsilane is an organosilicon compound ( silyl halide), with the formula , often abbreviated or TMSCl. It is a colourless volatile liquid that is stable in the absence of water. It is widely ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |