silyl enol ether on:
[Wikipedia]
[Google]
[Amazon]
In organosilicon chemistry, silyl enol ethers are a class of
 Alternatively, a rather exotic way of generating silyl enol ethers is via the Brook rearrangement of appropriate substrates.
Alternatively, a rather exotic way of generating silyl enol ethers is via the Brook rearrangement of appropriate substrates.



 Acyloins form upon
Acyloins form upon 



organic compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-co ...
s that share the common functional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
, composed of an enolate () bonded to a silane
Silane (Silicane) is an inorganic compound with chemical formula . It is a colorless, pyrophoric gas with a sharp, repulsive, pungent smell, somewhat similar to that of acetic acid. Silane is of practical interest as a precursor to elemental ...
() through its oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
end and an ethene group () as its carbon end. They are important intermediates in organic synthesis
Organic synthesis is a branch of chemical synthesis concerned with the construction of organic compounds. Organic compounds are molecules consisting of combinations of covalently-linked hydrogen, carbon, oxygen, and nitrogen atoms. Within the gen ...
.
Synthesis
Silyl enol ethers are generally prepared by reacting an enolizablecarbonyl
In organic chemistry, a carbonyl group is a functional group with the formula , composed of a carbon atom double bond, double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds (such a ...
compound with a silyl electrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively Electric charge, charged, have an ...
and a base, or just reacting an enolate with a silyl electrophile.Clayden, J., Greeves, N., & Warren, S. (2012). Silyl enol ethers. In ''Organic chemistry'' (Second ed., pp. 466-467). Oxford University Press. Since silyl electrophiles are hard and silicon-oxygen bonds are very strong, the oxygen (of the carbonyl compound or enolate) acts as the nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
to form a Si-O single bond.
The most commonly used silyl electrophile is trimethylsilyl chloride. To increase the rate of reaction, trimethylsilyl triflate may also be used in the place of trimethylsilyl chloride as a more electrophilic substrate.
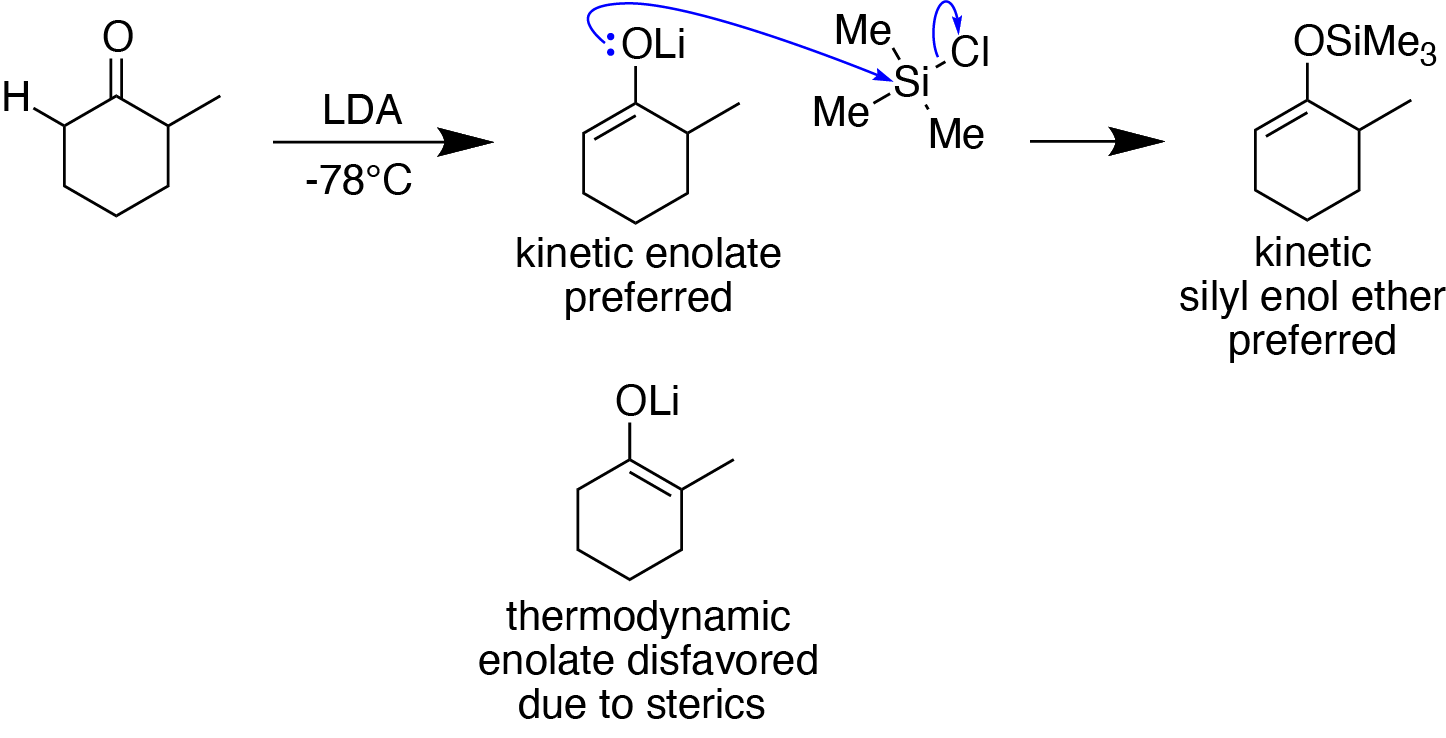
When using an unsymmetrical enolizable carbonyl compound as a substrate, the choice of reaction conditions can help control whether the kinetic or thermodynamic silyl enol ether is preferentially formed.Chan, T.-H. (1991). Formation and Addition Reactions of Enol Ethers. In ''Comprehensive Organic Synthesis'' (pp. 595–628). Elsevier. doi:10.1016/B978-0-08-052349-1.00042-1 For instance, when using lithium diisopropylamide (LDA), a strong and sterically hindered base, at low temperature (e.g., −78°C), the kinetic silyl enol ether (with a less substituted double bond) preferentially forms due to sterics.Clayden, J., Greeves, N., & Warren, S. (2012). Kinetically controlled enolate formation. In ''Organic chemistry'' (Second ed., pp. 600-601). Oxford University Press. When using triethylamine
Triethylamine is the chemical compound with the formula N(CH2CH3)3, commonly abbreviated Et3N. Like triethanolamine and the tetraethylammonium ion, it is often abbreviated TEA. It is a colourless volatile liquid with a strong fishy odor remini ...
, a weak base, the thermodynamic silyl enol ether (with a more substituted double bond) is preferred.

 Alternatively, a rather exotic way of generating silyl enol ethers is via the Brook rearrangement of appropriate substrates.
Alternatively, a rather exotic way of generating silyl enol ethers is via the Brook rearrangement of appropriate substrates.
Reactions
General reaction profile
Silyl enol ethers are neutral, mild nucleophiles (milder thanenamine
An enamine is an unsaturated compound derived by the condensation of an aldehyde or ketone with a secondary amine. Enamines are versatile intermediates.
The word "enamine" is derived from the affix ''en''-, used as the suffix of alkene, and the r ...
s) that react with good electrophiles such as aldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
s (with Lewis acid catalysis) and carbocation
Carbocation is a general term for ions with a positively charged carbon atom. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further ...
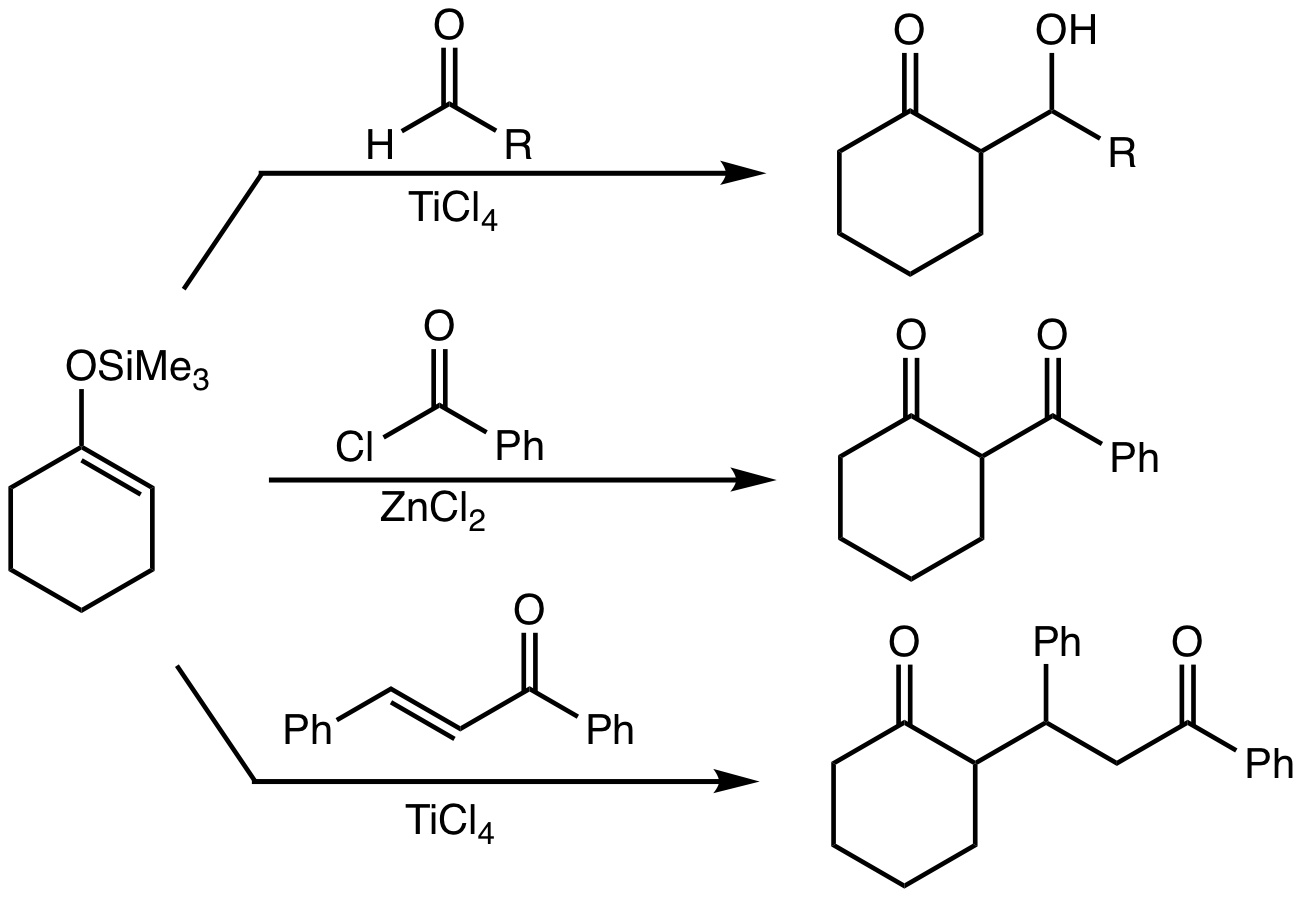
s.Clayden, J., Greeves, N., & Warren, S. (2012). Silyl enol ethers in aldol reactions. In ''Organic chemistry'' (Second ed., pp. 626-627). Oxford University Press.Clayden, J., Greeves, N., & Warren, S. (2012). Silyl enol ethers are alkylated by SN1-reactive electrophiles in the presence of Lewis acid. In ''Organic chemistry'' (Second ed., p. 595). Oxford University Press.Clayden, J., Greeves, N., & Warren, S. (2012). Conjugate addition of silyl enol ethers leads to the silyl enol ether of the product. In ''Organic chemistry'' (Second ed., pp. 608-609). Oxford University Press. Silyl enol ethers are stable enough to be isolated, but are usually used immediately after synthesis.
Generation of lithium enolate
Lithium enolates, one of the precursors to silyl enol ethers, can also be generated from silyl enol ethers usingmethyllithium
Methyllithium is the simplest organolithium reagent, with the empirical formula LiCH3. This s-block organometallic compound adopts an oligomeric structure both in solution and in the solid state. This highly reactive compound, invariably used i ...
.House, H. O., Gall, M., & Olmstead, H. D. (1971). Chemistry of carbanions. XIX. Alkylation of enolates from unsymmetrical ketones. ''The Journal of Organic Chemistry'', ''36''(16), 2361–2371. doi:10.1021/jo00815a037 The reaction occurs via nucleophilic substitution
In chemistry, a nucleophilic substitution (SN) is a class of chemical reactions in which an electron-rich chemical species (known as a nucleophile) replaces a functional group within another electron-deficient molecule (known as the electrophile) ...
at the silicon of the silyl enol ether, producing the lithium enolate and tetramethylsilane
Tetramethylsilane (abbreviated as TMS) is the organosilicon compound with the formula Si(CH3)4. It is the simplest tetraorganosilane. Like all silanes, the TMS framework is tetrahedral. TMS is a building block in organometallic chemistry but als ...
.

C–C bond formation
Silyl enol ethers are used in many reactions resulting inalkylation Alkylation is a chemical reaction that entails transfer of an alkyl group. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effecting al ...
, e.g., Mukaiyama aldol addition, Michael reactions, and Lewis-acid-catalyzed reactions with SN1-reactive electrophiles (e.g., tertiary
Tertiary (from Latin, meaning 'third' or 'of the third degree/order..') may refer to:
* Tertiary period, an obsolete geologic period spanning from 66 to 2.6 million years ago
* Tertiary (chemistry), a term describing bonding patterns in organic ch ...
, allylic
In organic chemistry, an allyl group is a substituent with the structural formula . It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . In 1844, Theodor Wertheim isolat ...
, or benzylic alkyl halides). Alkylation of silyl enol ethers is especially efficient with tertiary alkyl halides, which form stable carbocations in the presence of Lewis acids like or .



Halogenation and oxidations
Halogenation of silyl enol ethers gives haloketones.Clayden, J., Greeves, N., & Warren, S. (2012). Reactions of silyl enol ethers with halogen and sulfur electrophiles. In ''Organic chemistry'' (Second ed., pp. 469-470). Oxford University Press. Acyloins form upon
Acyloins form upon organic oxidation
Organic reductions or organic oxidations or organic redox reactions are redox reactions that take place with organic compounds. In organic chemistry oxidations and reductions are different from ordinary redox reactions, because many reactions carr ...
with an electrophilic source of oxygen such as an oxaziridine or mCPBA.
In the Saegusa–Ito oxidation, certain silyl enol ethers are oxidized to enones with palladium(II) acetate
Palladium is a chemical element; it has symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1802 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas (formally 2 Pallas), wh ...
.
:Sulfenylation
Reacting a silyl enol ether with PhSCl, a good and soft electrophile, provides a carbonyl compound sulfenylated at analpha carbon
In the nomenclature of organic chemistry, a locant is a term to indicate the position of a functional group or substituent within a molecule.
Numeric locants
The International Union of Pure and Applied Chemistry (IUPAC) recommends the use of n ...
. In this reaction, the trimethylsilyl
A trimethylsilyl group (abbreviated TMS) is a functional group in organic chemistry. This group consists of three methyl groups bonded to a silicon atom minus;Si(CH3)3 which is in turn bonded to the rest of a molecule. This structural group i ...
group of the silyl enol ether is removed by the chloride
The term chloride refers to a compound or molecule that contains either a chlorine anion (), which is a negatively charged chlorine atom, or a non-charged chlorine atom covalently bonded to the rest of the molecule by a single bond (). The pr ...
ion released from the PhSCl upon attack of its electrophilic sulfur atom.

Hydrolysis
Hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
of a silyl enol ether results in the formation of a carbonyl compound and a disiloxane.Clayden, J., Greeves, N., & Warren, S. (2012). Hydrolysis of enol ethers. In ''Organic chemistry'' (Second ed., pp. 468-469). Oxford University Press. In this reaction, water acts as an oxygen nucleophile and attacks the silicon of the silyl enol ether. This leads to the formation of the carbonyl compound and a trimethylsilanol
Trimethylsilanol (TMS) is an organosilicon compound with the formula . The Si centre bears three methyl groups and one hydroxyl group. It is a colourless volatile liquid.Paul D. Lickiss: ''The Synthesis and Structure of Organosilanols'', Advances i ...
intermediate that undergoes nucleophilic substitution at silicon (by another trimethylsilanol) to give the disiloxane.

Ring contraction
Cyclic silyl enol ethers undergo regiocontrolled one-carbon ring contractions.Mitcheltree, M. J.; Konst, Z. A.; Herzon, S. B. ''Tetrahedron'' 2013, ''69'', 5634. These reactions employ electron-deficient sulfonyl azides, which undergo chemoselective, uncatalyzed +2cycloaddition to the silyl enol ether, followed by loss of dinitrogen, and alkyl migration to give ring-contracted products in good yield. These reactions may be directed by substrate stereochemistry, giving rise to stereoselective ring-contracted product formation.Silyl ketene acetals
Silyl enol ethers ofester
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distin ...
s () or carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an Substituent, R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl ...
s () are called silyl ketene acetals and have the general structure . These compounds are more nucleophilic than the silyl enol ethers of ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
s ({{chem2, >C\dO).

References