|
Allylic
In organic chemistry, an allyl group is a substituent with the structural formula . It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . In 1844, Theodor Wertheim isolated an allyl derivative from garlic oil and named it "". The term allyl applies to many compounds related to , some of which are of practical or of everyday importance, for example, allyl chloride. Allylation is any chemical reaction that adds an allyl group to a substrate. Nomenclature A site adjacent to the unsaturated carbon atom is called the allylic position or allylic site. A group attached at this site is sometimes described as allylic. Thus, "has an allylic hydroxyl group". Allylic C−H bonds are about 15% weaker than the C−H bonds in ordinary sp3 carbon centers and are thus more reactive. Benzylic and allylic are related in terms of structure, bond strength, and reactivity. Other reactions that tend to occur with al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tsuji–Trost Reaction
The Tsuji–Trost reaction (also called the Trost allylic alkylation or allylic alkylation) is a palladium-catalysed substitution reaction involving a substrate that contains a leaving group in an allylic position. The palladium catalyst first coordinates with the allyl group and then undergoes oxidative addition, forming the -allyl complex. This transition metal allyl complex, allyl complex can then be attacked by a nucleophile, resulting in the substituted product. This work was first pioneered by Jirō Tsuji in 1965 and, later, adapted by Barry Trost in 1973 with the introduction of phosphine ligands. The scope of this reaction has been expanded to many different carbon, nitrogen, and oxygen-based nucleophiles, many different leaving groups, many different phosphorus, nitrogen, and sulfur-based ligands, and many different metals (although palladium is still preferred). The introduction of phosphine ligands led to improved reactivity and numerous asymmetric allylic alkylation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allyl
In organic chemistry, an allyl group is a substituent with the structural formula . It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . In 1844, Theodor Wertheim isolated an allyl derivative from garlic oil and named it "". The term allyl applies to many compounds related to , some of which are of practical or of everyday importance, for example, allyl chloride. Allylation is any chemical reaction that adds an allyl group to a Substrate (chemistry), substrate. Nomenclature A site adjacent to the unsaturated carbon atom is called the allylic position or allylic site. A group attached at this site is sometimes described as allylic. Thus, "has an allylic hydroxyl group". Allylic Carbon–hydrogen bond, C−H bonds are about 15% weaker than the C−H bonds in ordinary Orbital hybridisation, sp3 carbon centers and are thus more reactive. Benzylic and allylic are related in terms of structure, bond strength ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allylic Oxidation
In organic chemistry, an allyl group is a substituent with the structural formula . It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . In 1844, Theodor Wertheim isolated an allyl derivative from garlic oil and named it "". The term allyl applies to many compounds related to , some of which are of practical or of everyday importance, for example, allyl chloride. Allylation is any chemical reaction that adds an allyl group to a substrate. Nomenclature A site adjacent to the unsaturated carbon atom is called the allylic position or allylic site. A group attached at this site is sometimes described as allylic. Thus, "has an allylic hydroxyl group". Allylic C−H bonds are about 15% weaker than the C−H bonds in ordinary sp3 carbon centers and are thus more reactive. Benzylic and allylic are related in terms of structure, bond strength, and reactivity. Other reactions that tend to occur with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vinyl Group
In organic chemistry, a vinyl group (abbr. Vi; IUPAC name: ethenyl group) is a functional group with the formula . It is the ethylene (IUPAC name: ethene) molecule () with one fewer hydrogen atom. The name is also used for any compound containing that group, namely where R is any other group of atoms. An industrially important example is vinyl chloride, precursor to PVC, a plastic commonly known as ''vinyl''. Vinyl is one of the alkenyl functional groups. On a carbon skeleton, sp2-hybridized carbons or positions are often called vinylic. Allyls, acrylates and styrenics contain vinyl groups. (A styrenic crosslinker with two vinyl groups is called '' divinyl benzene''.) Vinyl polymers Vinyl groups can polymerize with the aid of a radical initiator or a catalyst, forming vinyl polymers. Vinyl polymers contain no vinyl groups. Instead they are saturated. The following table gives some examples of vinyl polymers. Synthesis and reactivity Vinyl derivatives are alke ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroperoxide
Hydroperoxides or peroxols are Chemical compound, compounds of the form ROOH, where R stands for any group, typically Organic compound, organic, which contain the hydroperoxy functional group (). Hydroperoxide also refers to the hydroperoxide anion () and its Salt (chemistry), salts, and the neutral hydroperoxyl, hydroperoxyl radical (•OOH) consist of an unbond hydroperoxy group. When R is organic, the compounds are called organic hydroperoxides. Such compounds are a subset of organic peroxides, which have the formula ROOR. Organic hydroperoxides can either intentionally or unintentionally initiate explosive polymerisation in materials with saturated bond, saturated chemical bonds. Properties The bond length in peroxides is about 1.45 Ångström, Å, and the angles (R = Hydrogen, H, Carbon, C) are about 110° (water-like). Characteristically, the dihedral angles are about 120°. The bond is relatively weak, with a bond dissociation energy of , less than half the strength ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bond Dissociation Energy
The bond-dissociation energy (BDE, ''D''0, or ''DH°'') is one measure of the strength of a chemical bond . It can be defined as the standard enthalpy change when is cleaved by homolysis to give fragments A and B, which are usually radical species. The enthalpy change is temperature-dependent, and the bond-dissociation energy is often defined to be the enthalpy change of the homolysis at 0 K (absolute zero), although the enthalpy change at 298 K ( standard conditions) is also a frequently encountered parameter. As a typical example, the bond-dissociation energy for one of the C−H bonds in ethane () is defined as the standard enthalpy change of the process : , : ''DH''°298() = Δ''H°'' = 101.1(4) kcal/mol = 423.0 ± 1.7 kJ/mol = 4.40(2) eV (per bond). To convert a molar BDE to the energy needed to dissociate the bond ''per molecule'', the conversion factor 23.060 kcal/mol (96.485 kJ/mol) for each eV can be used. A variety of experi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Jasmonate
Jasmonate (JA) and its derivatives are lipid-based plant hormones that regulate a wide range of processes in plants, ranging from growth and photosynthesis to reproductive development. In particular, JAs are critical for plant defense against herbivory and plant responses to poor environmental conditions and other kinds of abiotic and biotic challenges. Some JAs can also be released as volatile organic compounds (VOCs) to permit communication between plants in anticipation of mutual dangers. History The isolation of methyl jasmonate (MeJA) from jasmine oil derived from '' Jasminum grandiflorum'' led to the discovery of the molecular structure of jasmonates and their name in 1962 while jasmonic acid itself was isolated from '' Lasiodiplodia theobromae'' by Alderidge et al in 1971. Biosynthesis Biosynthesis is reviewed by Acosta and Farmer 2010, Wasternack and Hause 2013, and Wasternack and Song 2017. Jasmonates (JA) are oxylipins, i.e. derivatives of oxygenated fatty acid. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lipid Peroxidation
Lipid peroxidation, or lipid oxidation, is a complex chemical process that leads to oxidative degradation of lipids, resulting in the formation of peroxide and hydroperoxide derivatives.{{Cite journal , last1=Ayala , first1=Antonio , last2=Muñoz , first2=Mario F. , last3=Argüelles , first3=Sandro , date=2014 , title=Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal , journal=Oxidative Medicine and Cellular Longevity , language=en , volume=2014 , pages=1–31 , doi=10.1155/2014/360438 , doi-access=free , issn=1942-0900 , pmc=4066722 , pmid=24999379 It occurs when free radicals, specifically reactive oxygen species (ROS), interact with lipids within cell membranes, typically polyunsaturated fatty acids (PUFAs) as they have carbon–carbon double bonds. This reaction leads to the formation of lipid radicals, collectively referred to as lipid peroxides or lipid oxidation products (LOPs), which in turn react with other ox ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
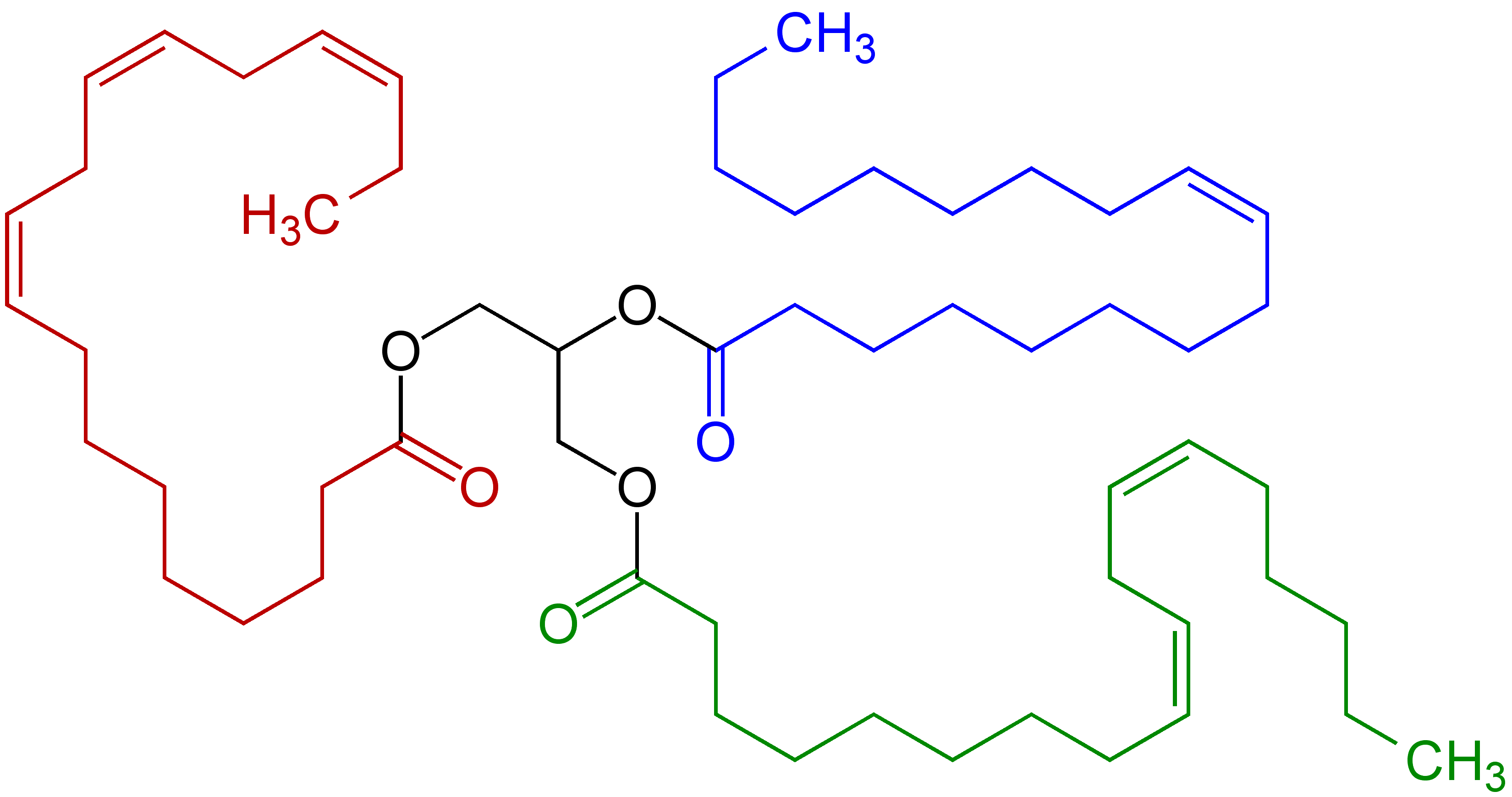
Arachidonic Acid
Arachidonic acid (AA, sometimes ARA) is a polyunsaturated omega−6 fatty acid 20:4(ω−6), or 20:4(5,8,11,14). It is a precursor in the formation of leukotrienes, prostaglandins, and thromboxanes. Together with omega−3 fatty acids and other omega−6 fatty acids, arachidonic acid provides energy for body functions, contributes to cell membrane structure, and participates in the synthesis of eicosanoids, which have numerous roles in physiology as signaling molecules. Its name derives from the ancient Greek neologism ''arachis'' 'peanut', although peanut oil does not contain any arachidonic acid. Arachidonate is the name of the derived carboxylate anion ( conjugate base of the acid), salts, and some esters. Chemistry In chemical structure, arachidonic acid is a carboxylic acid with a 20-carbon chain and four '' cis''- double bonds; the first double bond is located at the sixth carbon from the omega end. Some chemistry sources define 'arachidonic acid' to designa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Linolenic Acid
Linolenic acid is a type of naturally-occurring fatty acid. It can refer to either of two octadecatrienoic acids (i.e. with an 18-carbon chain and three double bonds, which are found in the '' cis'' configuration), or a mixture of the two. Linolenate (in the form of triglyceride esters of linolenic acid) is often found in vegetable oils; traditionally, such fatty acylates are reported as the fatty acids: *α-Linolenic acid, an omega-3 (n-3) fatty acid * γ-Linolenic acid, an omega-6 (n-6) fatty acid See also * Linoleic acid Linoleic acid (LA) is an organic compound with the formula . Both alkene groups () are ''cis''. It is a fatty acid sometimes denoted 18:2 (n−6) or 18:2 ''cis''-9,12. A linoleate is a salt or ester of this acid. Linoleic acid is a polyunsat ..., the similarly named essential fatty acid References {{chemistry index Alkenoic acids Aromatase inhibitors Fatty acids Essential fatty acids Essential nutrients ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Linoleic Acid
Linoleic acid (LA) is an organic compound with the formula . Both alkene groups () are ''cis''. It is a fatty acid sometimes denoted 18:2 (n−6) or 18:2 ''cis''-9,12. A linoleate is a salt or ester of this acid. Linoleic acid is a polyunsaturated, omega−6 fatty acid. It is a colorless liquid that is virtually insoluble in water but soluble in many organic solvents. It typically occurs in nature as a triglyceride (ester of glycerin) rather than as a free fatty acid. It is one of two essential fatty acids for humans, who must obtain it through their diet, and the most essential, because the body uses it as a base to make the others. The word "linoleic" derives , reflecting the fact that it was first isolated from linseed oil. History In 1844, F. Sacc, working at the laboratory of Justus von Liebig Justus ''Freiherr'' von Liebig (12 May 1803 – 18 April 1873) was a Germans, German scientist who made major contributions to the theory, practice, and pedagogy of che ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyunsaturated Fatty Acid
In biochemistry and nutrition, a polyunsaturated fat is a fat that contains a polyunsaturated fatty acid (abbreviated PUFA), which is a subclass of fatty acid characterized by a backbone with two or more carbon–carbon double bonds. Some polyunsaturated fatty acids are essentials. Polyunsaturated fatty acids are precursors to and are derived from polyunsaturated fats, which include drying oils. Nomenclature The position of the carbon-carbon double bonds in carboxylic acid chains in fats is designated by Greek letters. The carbon atom closest to the carboxyl group is the ''alpha'' carbon, the next carbon is the ''beta'' carbon and so on. In fatty acids the carbon atom of the methyl group at the end of the hydrocarbon chain is called the ''omega'' carbon because ''omega'' is the last letter of the Greek alphabet. Omega-3 fatty acids have a double bond three carbons away from the methyl carbon, whereas omega-6 fatty acids have a double bond six carbons away from the methyl ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |