Silyl on:
[Wikipedia]
[Google]
[Amazon]
Silylation is the introduction of one or more (usually) substituted silyl groups (R3Si) to a molecule. Silylations are core methods for production of organosilicon chemistry. Silanization, while similar to silylation, usually refers to attachment of silyl groups to solids. Silyl groups are commonly used for: alcohol protection, enolate trapping,
 The protection mechanism begins with the base deprotonating the alcohol group. Next, the deprotonated alcohol group attacks the silyl atom of the silyl halide compound. The halide acts as a leaving group and ends up in solution. A workup step follows to remove any excess base within the solution. The overall reaction scheme is as follows:
#
#
Other silylating agents include bis(trimethylsilyl)acetamide (BSA). The reaction of BSA with alcohols gives the corresponding trimethyl silyl ether, together with acetamide as a byproduct (Me = CH3):
:
The protection mechanism begins with the base deprotonating the alcohol group. Next, the deprotonated alcohol group attacks the silyl atom of the silyl halide compound. The halide acts as a leaving group and ends up in solution. A workup step follows to remove any excess base within the solution. The overall reaction scheme is as follows:
#
#
Other silylating agents include bis(trimethylsilyl)acetamide (BSA). The reaction of BSA with alcohols gives the corresponding trimethyl silyl ether, together with acetamide as a byproduct (Me = CH3):
:
 Deprotection with a fluoride ion occurs by an SN2 mechanism, followed by acidic workup to protonate the resulting alkoxide:
Deprotection of the alcohol can also be done using either Brønsted acids or Lewis acid conditions. Brønsted acids, like PyBr3 (pyridinium tribromide), deprotect the alcohol by acting as a proton donor.
Deprotection with a fluoride ion occurs by an SN2 mechanism, followed by acidic workup to protonate the resulting alkoxide:
Deprotection of the alcohol can also be done using either Brønsted acids or Lewis acid conditions. Brønsted acids, like PyBr3 (pyridinium tribromide), deprotect the alcohol by acting as a proton donor.
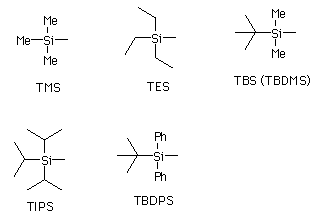 Sterically bulkier alkyl substituents tend to decrease the reactivity of the silyl group. Consequently, bulky substituents increase the silyl group's protective abilities. To add bulkier alkyl silyls, more strenuous conditions are required for alcohol protection. As bulkier groups require more strenuous conditions to add, they also require more strenuous conditions to remove. Additionally, bulkier silyl groups are more selective for the type of alcohols they react with, resulting in a preference for primary alcohols over secondary alcohols. Thus, silyl groups such as TBDMS and TIPS can be used to selectively protect primary alcohols over secondary alcohols.
In acidic conditions, alkyl substituents acting as electron withdrawing groups decrease the reaction rate. As bulker silyl groups are more likely to be electron withdrawing, it is easier to differentiate between less and more bulky silyl groups. Therefore, acidic deprotection occurs fastest for less sterically bulky alkyl silyl groups. In basic conditions, alkyl substituents acting as electron donating groups decrease reaction rate.
Sterically bulkier alkyl substituents tend to decrease the reactivity of the silyl group. Consequently, bulky substituents increase the silyl group's protective abilities. To add bulkier alkyl silyls, more strenuous conditions are required for alcohol protection. As bulkier groups require more strenuous conditions to add, they also require more strenuous conditions to remove. Additionally, bulkier silyl groups are more selective for the type of alcohols they react with, resulting in a preference for primary alcohols over secondary alcohols. Thus, silyl groups such as TBDMS and TIPS can be used to selectively protect primary alcohols over secondary alcohols.
In acidic conditions, alkyl substituents acting as electron withdrawing groups decrease the reaction rate. As bulker silyl groups are more likely to be electron withdrawing, it is easier to differentiate between less and more bulky silyl groups. Therefore, acidic deprotection occurs fastest for less sterically bulky alkyl silyl groups. In basic conditions, alkyl substituents acting as electron donating groups decrease reaction rate.


Identification of Silylation Artifacts in Derivatization Reactions for Gas Chromatography
Desilylation methods
Chemical processes Organosilicon compounds
gas chromatography
Gas chromatography (GC) is a common type of chromatography used in analytical chemistry for Separation process, separating and analyzing compounds that can be vaporized without Chemical decomposition, decomposition. Typical uses of GC include t ...
, electron-impact mass spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a ''mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is used ...
(EI-MS), and coordinating with metal complexes.
Protection Chemistry
Protection
Silylation is often used to protect alcohols, as well as amines, carboxylic acids, and terminal alkynes. The products after silylation, namely silyl ethers and silyl amines, are resilient toward basic conditions. Protection is typically done by reacting the functional group with a silyl halide by anSN2 reaction
The bimolecular nucleophilic substitution (SN2) is a type of reaction mechanism that is common in organic chemistry. In the SN2 reaction, a strong nucleophile forms a new bond to an sp3-hybridised carbon atom via a backside attack, all while t ...
mechanism, typically in the presence of base.
 The protection mechanism begins with the base deprotonating the alcohol group. Next, the deprotonated alcohol group attacks the silyl atom of the silyl halide compound. The halide acts as a leaving group and ends up in solution. A workup step follows to remove any excess base within the solution. The overall reaction scheme is as follows:
#
#
Other silylating agents include bis(trimethylsilyl)acetamide (BSA). The reaction of BSA with alcohols gives the corresponding trimethyl silyl ether, together with acetamide as a byproduct (Me = CH3):
:
The protection mechanism begins with the base deprotonating the alcohol group. Next, the deprotonated alcohol group attacks the silyl atom of the silyl halide compound. The halide acts as a leaving group and ends up in solution. A workup step follows to remove any excess base within the solution. The overall reaction scheme is as follows:
#
#
Other silylating agents include bis(trimethylsilyl)acetamide (BSA). The reaction of BSA with alcohols gives the corresponding trimethyl silyl ether, together with acetamide as a byproduct (Me = CH3):
:
Deprotection
Due to the strength of the Si-F bond, fluoride salts are commonly used as a deprotecting agent of silyl groups. The primary fluorous deprotecting agent istetra-n-butylammonium fluoride
Tetra-''n''-butylammonium fluoride, commonly abbreviated to TBAF and ''n''-Bu4NF, is a quaternary ammonium salt with the chemical formula (CH3CH2CH2CH2)4N+F−. It is commercially available as the white solid trihydrate and as a solution in tetra ...
(TBAF), as its aliphatic chains in help incorporate the fluoride ion into organic solvents.
 Deprotection with a fluoride ion occurs by an SN2 mechanism, followed by acidic workup to protonate the resulting alkoxide:
Deprotection of the alcohol can also be done using either Brønsted acids or Lewis acid conditions. Brønsted acids, like PyBr3 (pyridinium tribromide), deprotect the alcohol by acting as a proton donor.
Deprotection with a fluoride ion occurs by an SN2 mechanism, followed by acidic workup to protonate the resulting alkoxide:
Deprotection of the alcohol can also be done using either Brønsted acids or Lewis acid conditions. Brønsted acids, like PyBr3 (pyridinium tribromide), deprotect the alcohol by acting as a proton donor.Modifying Silyl Reactivity
 Sterically bulkier alkyl substituents tend to decrease the reactivity of the silyl group. Consequently, bulky substituents increase the silyl group's protective abilities. To add bulkier alkyl silyls, more strenuous conditions are required for alcohol protection. As bulkier groups require more strenuous conditions to add, they also require more strenuous conditions to remove. Additionally, bulkier silyl groups are more selective for the type of alcohols they react with, resulting in a preference for primary alcohols over secondary alcohols. Thus, silyl groups such as TBDMS and TIPS can be used to selectively protect primary alcohols over secondary alcohols.
In acidic conditions, alkyl substituents acting as electron withdrawing groups decrease the reaction rate. As bulker silyl groups are more likely to be electron withdrawing, it is easier to differentiate between less and more bulky silyl groups. Therefore, acidic deprotection occurs fastest for less sterically bulky alkyl silyl groups. In basic conditions, alkyl substituents acting as electron donating groups decrease reaction rate.
Sterically bulkier alkyl substituents tend to decrease the reactivity of the silyl group. Consequently, bulky substituents increase the silyl group's protective abilities. To add bulkier alkyl silyls, more strenuous conditions are required for alcohol protection. As bulkier groups require more strenuous conditions to add, they also require more strenuous conditions to remove. Additionally, bulkier silyl groups are more selective for the type of alcohols they react with, resulting in a preference for primary alcohols over secondary alcohols. Thus, silyl groups such as TBDMS and TIPS can be used to selectively protect primary alcohols over secondary alcohols.
In acidic conditions, alkyl substituents acting as electron withdrawing groups decrease the reaction rate. As bulker silyl groups are more likely to be electron withdrawing, it is easier to differentiate between less and more bulky silyl groups. Therefore, acidic deprotection occurs fastest for less sterically bulky alkyl silyl groups. In basic conditions, alkyl substituents acting as electron donating groups decrease reaction rate.
Enolate Trapping
Silylation can also be used to trap reactive compounds for isolation or identification. A common example of this is by trapping reactive enolates into silyl enol ethers, which represent reactive tautomers of many carbonyl compounds. The original enolate can be reformed upon reaction with an organolithium, or other strong base.
Applications in Analysis
The introduction of a silyl group(s) gives derivatives of enhanced volatility, making the derivatives suitable for analysis bygas chromatography
Gas chromatography (GC) is a common type of chromatography used in analytical chemistry for Separation process, separating and analyzing compounds that can be vaporized without Chemical decomposition, decomposition. Typical uses of GC include t ...
and electron-impact mass spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a ''mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is used ...
(EI-MS). For EI-MS, the silyl derivatives give more favorable diagnostic fragmentation patterns of use in structure investigations, or characteristic ions of use in trace analyses employing selected ion monitoring and related techniques.
Of metals

Coordination complex
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of chemical bond, bound molecules or ions, that are in turn known as ' ...
es with silyl ligands are well known. An early example is CpFe(CO)2Si(CH3)3, prepared by silylation of CpFe(CO)2Na with trimethylsilyl chloride. Typical routes include oxidative addition of Si-H bonds to low-valent metals. Metal silyl complexes are intermediates in hydrosilation, a process used to make organosilicon compounds on both laboratory and commercial scales.
See also
* Silyl ether * HydrosilylationReferences
{{reflistExternal links
Identification of Silylation Artifacts in Derivatization Reactions for Gas Chromatography
Desilylation methods
Chemical processes Organosilicon compounds