TCID50 on:
[Wikipedia]
[Google]
[Amazon]
Virus quantification is counting or calculating the number of
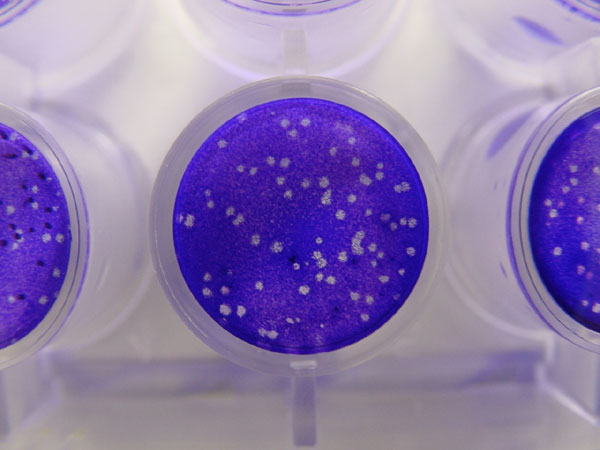 Plaque-based assays are a commonly used method to determine virus concentration in terms of
Plaque-based assays are a commonly used method to determine virus concentration in terms of
 The focus forming assay (FFA) is a variation of the plaque assay, but instead of depending on cell lysis in order to detect plaque formation, the FFA employs
The focus forming assay (FFA) is a variation of the plaque assay, but instead of depending on cell lysis in order to detect plaque formation, the FFA employs
 Enzyme-linked immunosorbent assay (
Enzyme-linked immunosorbent assay (
virus
A virus is a submicroscopic infectious agent that replicates only inside the living Cell (biology), cells of an organism. Viruses infect all life forms, from animals and plants to microorganisms, including bacteria and archaea. Viruses are ...
particles (virions) in a sample to determine the virus concentration. It is used in both research and development
Research and development (R&D or R+D), known in some countries as OKB, experiment and design, is the set of innovative activities undertaken by corporations or governments in developing new services or products. R&D constitutes the first stage ...
(R&D) in academic and commercial laboratories as well as in production situations where the quantity of virus at various steps is an important variable that must be monitored. For example, the production of virus-based vaccines
A vaccine is a biological preparation that provides active acquired immunity to a particular infectious or malignant disease. The safety and effectiveness of vaccines has been widely studied and verified. A vaccine typically contains an ag ...
, recombinant proteins using viral vectors, and viral antigens
In immunology, an antigen (Ag) is a molecule, moiety, foreign particulate matter, or an allergen, such as pollen, that can bind to a specific antibody or T-cell receptor. The presence of antigens in the body may trigger an immune response.
An ...
all require virus quantification to continually monitor and/or modify the process in order to optimize product quality and production yields and to respond to ever changing demands and applications. Other examples of specific instances where viruses need to be quantified include clone screening, multiplicity of infection
In microbiology, the multiplicity of infection or MOI is the ratio of agents (e.g. phage or more generally virus, bacteria) to infection targets (e.g. cell). For example, when referring to a group of cells inoculated with virus particles, the MO ...
(MOI) optimization, and adaptation of methods to cell culture
Cell culture or tissue culture is the process by which cell (biology), cells are grown under controlled conditions, generally outside of their natural environment. After cells of interest have been Cell isolation, isolated from living tissue, ...
.
There are many ways to categorize virus quantification methods. Here, the methods are grouped according to what is being measured and in what biological context. For example, cell-based assays typically measure infectious units (active virus). Other methods may measure the concentration of viral proteins, DNA, RNA, or molecular particles, but not necessarily measure infectivity. Each method has its own advantages and disadvantages, which often determines which method is used for specific applications.
Cell-based assays
Plaque assay
 Plaque-based assays are a commonly used method to determine virus concentration in terms of
Plaque-based assays are a commonly used method to determine virus concentration in terms of infectious dose The concept of a minimal infective dose (MID), also known as the infectious dose, has traditionally been used for infectious microorganisms that contaminate foods. MID was defined as the number of microorganisms ingested (the dose) from which a pat ...
. Plaque assays determine the number of plaque forming units (PFU) in a virus sample, which is one measure of virus quantity. This assay is based on a microbiological method conducted in petri dishes or multi-well cell culture
Cell culture or tissue culture is the process by which cell (biology), cells are grown under controlled conditions, generally outside of their natural environment. After cells of interest have been Cell isolation, isolated from living tissue, ...
plates. Specifically, a confluent monolayer of host
A host is a person responsible for guests at an event or for providing hospitality during it.
Host may also refer to:
Places
* Host, Pennsylvania, a village in Berks County
* Host Island, in the Wilhelm Archipelago, Antarctica
People
* ...
cells is infected by applying a sample containing the virus at varying dilutions and then covered with a semi-solid medium
Medium may refer to:
Aircraft
*Medium bomber, a class of warplane
* Tecma Medium, a French hang glider design Arts, entertainment, and media Films
* ''The Medium'' (1921 film), a German silent film
* ''The Medium'' (1951 film), a film vers ...
, such as agar
Agar ( or ), or agar-agar, is a jelly-like substance consisting of polysaccharides obtained from the cell walls of some species of red algae, primarily from " ogonori" and " tengusa". As found in nature, agar is a mixture of two components, t ...
or carboxymethyl cellulose, to prevent the virus infection from spreading indiscriminately, as would occur in a liquid medium. A viral plaque
A viral plaque is a visible structure formed after introducing a viral sample to a cell culture grown on some nutrient medium. The virus will replicate and spread, generating regions of cell destruction known as plaques. For example, Vero cell o ...
is formed after a virus infects a cell within the fixed cell monolayer. The virus-infected cell will lyse and spread the infection to adjacent cells, where the infection-to-lysis cycle is repeated. This will create an area of infected, lysed cells (viral plaque) surrounded by uninfected, intact cells. The plaque can be seen with an optical microscope or visually using cell staining techniques (e.g., staining with a crystal violet
Crystal violet or gentian violet, also known as methyl violet 10B or hexamethyl pararosaniline chloride, is a triphenylmethane, triarylmethane dye used as a histological stain and in Gram staining, Gram's method of classifying bacteria. Crystal ...
solution to visualize intact vs. lysed cells). Plaque formation can take 3–14 days, depending on the virus being analyzed. Plaques are generally counted manually, and the plaque count, in combination with the dilution factor of the infection solution (the sample initially applied to the cells), is used to calculate the number of plaque forming units per sample unit volume (PFU/mL). The PFU/mL number represents the concentration of infectious virus particles within the sample and is based on the assumption that each plaque formed is representative of an initial infection by one infectious virus particle.
Focus forming assay (FFA)
 The focus forming assay (FFA) is a variation of the plaque assay, but instead of depending on cell lysis in order to detect plaque formation, the FFA employs
The focus forming assay (FFA) is a variation of the plaque assay, but instead of depending on cell lysis in order to detect plaque formation, the FFA employs immunostaining
In biochemistry, immunostaining is any use of an antibody-based method to detect a specific protein in a sample. The term "immunostaining" was originally used to refer to the immunohistochemical staining of tissue sections, as first described by ...
techniques using fluorescently labeled antibodies specific for a viral antigen
In immunology, an antigen (Ag) is a molecule, moiety, foreign particulate matter, or an allergen, such as pollen, that can bind to a specific antibody or T-cell receptor. The presence of antigens in the body may trigger an immune response.
...
to detect infected host cells and infectious virus particles before an actual plaque is formed. The FFA is particularly useful for quantifying classes of viruses that do not lyse the cell membranes, as these viruses would not be amenable to the plaque assay. Like the plaque assay, host cell monolayers are infected with various dilutions of the virus sample and allowed to incubate for a relatively brief incubation period (e.g., 24–72 hours) under a semisolid overlay medium that restricts the spread of infectious virus, creating localized clusters (foci) of infected cells. Plates are subsequently probed with fluorescently labeled antibodies against a viral antigen, and fluorescence microscopy
A fluorescence microscope is an optical microscope that uses fluorescence instead of, or in addition to, scattering, reflection, and attenuation or absorption, to study the properties of organic or inorganic substances. A fluorescence micro ...
is used to count and quantify the number of foci. The FFA method typically yields results in less time than plaque assays or fifty-percent-tissue-culture-infective-dose (TCID50) assays (see below), but it can be more expensive in terms of required reagents and equipment. Assay completion time is also dependent on the size of area that the user is counting. A larger area will require more time but can provide a more accurate representation of the sample. Results of the FFA are expressed as focus forming units per milliliter, or FFU/mL.
TCID50 endpoint dilution assay
The TCID50 (50% tissue culture infectious dose) assay is the measure of infectious virus titer. This endpoint dilution assay quantifies the amount of virus required to kill 50% of infected hosts or to produce acytopathic effect
Cytopathic effect (abbreviated CPE) refers to structural changes in host cells that are caused by viral invasion. The infecting virus causes lysis of the host cell or when the cell dies without lysis due to an inability to replicate. If a virus c ...
in 50% of inoculated tissue culture cells. This assay may be more common in clinical research applications where the lethal dose of virus must be determined or if the virus does not form plaques. When used in the context of tissue culture, host cells are plated and serial dilutions of the virus are added. After incubation, the percentage of cell death (i.e. infected cells) is manually observed and recorded for each virus dilution, and results are used to mathematically calculate a TCID50 result. Due to distinct differences in assay methods and principles, TCID50 and pfu/mL or other infectivity assay results are not equivalent. This method can take up to a week due to cell infectivity time.
Two methods commonly used to calculate TCID50 (can also be used to calculate other types of 50% endpoint such EC50
]
Half maximal effective concentration (EC50) is a measure of the concentration of a drug, antibody or toxicant which induces a stimulus–response model, biological response halfway between the baseline and maximum after a specified exposure tim ...
, IC50, and LD50
In toxicology, the median lethal dose, LD50 (abbreviation for "lethal dose, 50%"), LC50 (lethal concentration, 50%) or LCt50 is a toxic unit that measures the lethal dose of a given substance. The value of LD50 for a substance is the dose requ ...
) are:
* Spearman–Kärber
* Reed–Muench method
The ''theoretical'' relationship between TCID50 and PFU is approximately 0.69 PFU = 1 TCID50 based on the Poisson distribution
In probability theory and statistics, the Poisson distribution () is a discrete probability distribution that expresses the probability of a given number of events occurring in a fixed interval of time if these events occur with a known const ...
, a probability distribution
In probability theory and statistics, a probability distribution is a Function (mathematics), function that gives the probabilities of occurrence of possible events for an Experiment (probability theory), experiment. It is a mathematical descri ...
which describes how many random events (virus particles) occurring at a known average rate (virus titer) are likely to occur in a fixed space (the amount of virus medium in a well). However, it must be emphasized that in practice, this relationship may not hold even for the same virus + cell combination, as the two types of assay are set up differently and virus infectivity is very sensitive to various factors such as cell age, overlay media, etc.
But the following reference defines the relationship differently:
From ATTC: "Assuming that the same cell system is used, that the virus forms plaques on those cells, and that no procedures are added which would inhibit plaque formation, 1 mL of virus stock would be expected to have about half of the number of plaque forming units (PFUs) as TCID50. This is only an estimate but is based on the rationale that the limiting dilution which would infect 50% of the cell layers challenged would often be expected to initially produce a single plaque in the cell layers which become infected. In some instances, two or more plaques might by chance form, and thus the actual number of PFUs should be determined experimentally.
"Mathematically, the expected PFUs would be somewhat greater than one-half the TCID50, since the negative tubes in the TCID50 represent zero plaque forming units and the positive tubes each represent one or more plaque forming units. A more precise estimate is obtained by applying the Poisson distribution. Where is the proportion of negative tubes and m is the mean number of infectious units per volume (PFU/ml), . For any titer expressed as a TCID50, . Thus and which is ~ 0.7.
"Therefore, one could multiply the TCID50 titer (per ml) by 0.7 to predict the mean number of PFU/ml. When actually applying such calculations, remember the calculated mean will only be valid if the changes in protocol required to visualize plaques do not alter the expression of infectious virus as compared with expression under conditions employed for TCID50.
"Thus as a working estimate, one can assume material with a TCID50 of 1 × 105 TCID50/mL will produce 0.7 × 105 PFUs/mL."
Protein and antibody-based assays
There are several variations of protein- and antibody-based virus quantification assays. In general, these methods quantify either the amount of all protein or the amount of a specific virus protein in the sample rather than the number of infected cells or virus particles. Quantification commonly relies oncolorimetric
Colorimetry is "the science and technology used to quantify and describe physically the human color perception".
It is similar to spectrophotometry, but is distinguished by its interest in reducing spectra to the physical correlates of color p ...
or fluorescence
Fluorescence is one of two kinds of photoluminescence, the emission of light by a substance that has absorbed light or other electromagnetic radiation. When exposed to ultraviolet radiation, many substances will glow (fluoresce) with colore ...
detection. Some assay variations quantify proteins directly in a sample, while other variations require host cell infection and incubation to allow virus growth prior to quantification. The variation used depends primarily on the amount of protein (i.e. viral protein) in the initial sample and the sensitivity of the assay itself. If incubation and virus growth are required, cell and/or virus lysis/digestion are often conducted prior to analysis. Most protein-based methods are relatively fast and sensitive but require quality standards for accurate calibration, and quantify protein, not actual virus particle concentrations. Below are specific examples of widely used protein-based assays.
Hemagglutination assay
The hemagglutination assay (HA) is a common non-fluorescence protein quantification assay specific forinfluenza
Influenza, commonly known as the flu, is an infectious disease caused by influenza viruses. Symptoms range from mild to severe and often include fever, runny nose, sore throat, muscle pain, headache, coughing, and fatigue. These sympto ...
. It relies on the fact that hemagglutinin
The term hemagglutinin (alternatively spelt ''haemagglutinin'', from the Greek , 'blood' + Latin , 'glue') refers to any protein that can cause red blood cells (erythrocytes) to clump together (" agglutinate") ''in vitro''. They do this by bindin ...
, a surface protein of influenza viruses, agglutinates red blood cells (i.e. causes red blood cells to clump together). In this assay, dilutions of an influenza sample are incubated with a 1% erythrocyte
Red blood cells (RBCs), referred to as erythrocytes (, with -''cyte'' translated as 'cell' in modern usage) in academia and medical publishing, also known as red cells, erythroid cells, and rarely haematids, are the most common type of blood ce ...
solution for one hour and the virus dilution at which agglutination first occurs is visually determined. The assay produces a result of hemagglutination units (HAU), with typical PFU to HAU ratios in the 106 range. This assay takes ~1–2 hours to complete.
The hemagglutination inhibition assay is a common variation of the HA assay used to measure flu-specific antibody levels in blood serum. In this variation, serum antibodies to the influenza virus will interfere with the virus attachment to red blood cells. Therefore, hemagglutination is inhibited when antibodies are present at a sufficient concentration.
Bicinchoninic acid (BCA) assay
The bicinchoninic acid assay (BCA; a.k.a. Smith assay) is based on a simplecolorimetric
Colorimetry is "the science and technology used to quantify and describe physically the human color perception".
It is similar to spectrophotometry, but is distinguished by its interest in reducing spectra to the physical correlates of color p ...
measurement and is a commonly used protein quantification assay. BCA is similar to the Lowry or Bradford
Bradford is a city status in the United Kingdom, city in West Yorkshire, England. It became a municipal borough in 1847, received a city charter in 1897 and, since the Local Government Act 1972, 1974 reform, the city status in the United Kingdo ...
protein assays. The BCA assay reagent was first developed and made commercially by Pierce Chemical Company (now owned by Thermo Fisher Scientific
Thermo Fisher Scientific Inc. is an American life science and clinical research company. It is a global supplier of analytical instruments, clinical development solutions, specialty diagnostics, laboratory, pharmaceutical and biotechnology s ...
) which held the patent until 2006.
In the BCA assay, a protein's peptide bonds quantitatively reduce Cu2+ to Cu1+, which produces a light blue color. BCA chelates Cu1+ at a 2:1 ratio resulting in a more intensely colored species that absorbs at562 nm. Absorbance
Absorbance is defined as "the logarithm of the ratio of incident to transmitted radiant power through a sample (excluding the effects on cell walls)". Alternatively, for samples which scatter light, absorbance may be defined as "the negative log ...
of a sample at 562 nm is used to determine the bulk protein concentration in the sample. Assay results are compared with known standard curves after analysis with a spectrophotometer or plate reader. Total assay time is 30 minutes to one hour. While this assay is ubiquitous and fast, it lacks specificity to viral proteins since it counts all protein in the sample. Thus the virus preparation to be quantified must contain very low levels of host cell proteins.
Enzyme-linked immunosorbent assay (ELISA)
 Enzyme-linked immunosorbent assay (
Enzyme-linked immunosorbent assay (ELISA
The enzyme-linked immunosorbent assay (ELISA) (, ) is a commonly used analytical biochemistry assay, first described by Eva Engvall and Peter Perlmann in 1971. The assay is a solid-phase type of enzyme immunoassay (EIA) to detect the presence of ...
) is an antibody
An antibody (Ab) or immunoglobulin (Ig) is a large, Y-shaped protein belonging to the immunoglobulin superfamily which is used by the immune system to identify and neutralize antigens such as pathogenic bacteria, bacteria and viruses, includin ...
-based assay that utilizes an antigen
In immunology, an antigen (Ag) is a molecule, moiety, foreign particulate matter, or an allergen, such as pollen, that can bind to a specific antibody or T-cell receptor. The presence of antigens in the body may trigger an immune response.
...
-specific antibody chemically linked to an enzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different mol ...
(or bound to a second antibody linked to an enzyme) to detect the presence of an unknown amount of the antigen (e.g., viral protein) in a sample. The antibody-antigen binding event is detected and/or quantified through the enzyme's ability to convert a substrate reagent to produce a detectable signal that can then be used to calculate the concentration of the target antigen in the sample. Horseradish peroxidase
The enzyme horseradish peroxidase (HRP), found in the roots of horseradish, is used extensively in biochemistry applications. It is a metalloenzyme with many isoforms, of which the most studied type is C. It catalyzes the oxidation of various or ...
(HRP) is a common enzyme utilized in ELISA schemes due to its ability to amplify signal and increase assay sensitivity.
There are many variations, or types of ELISA assays but they can generally be classified as either indirect, competitive
Competition is a rivalry where two or more parties strive for a common goal which cannot be shared: where one's gain is the other's loss (an example of which is a zero-sum game). Competition can arise between entities such as organisms, indi ...
, sandwich
A sandwich is a Dish (food), dish typically consisting variously of meat, cheese, sauces, and vegetables used as a filling between slices of bread, or placed atop a slice of bread; or, more generally, any dish in which bread serves as a ''co ...
or reverse.
Single radial immunodiffusion (SRID) assay
Single radial immunodiffusion assay (SRID), also known as the Mancini method, is a protein assay that detects the amount of specific viral antigen by immunodiffusion in a semi-solid medium (e.g. agar). The medium containsantiserum
In immunology, antiserum is a blood serum containing antibodies (either monoclonal or polyclonal) that is used to spread passive immunity to many diseases via blood donation ( plasmapheresis). For example, convalescent serum, or passive ant ...
specific to the antigen of interest and the antigen is placed in the center of the disc. As the antigen diffuses into the medium it creates a precipitate ring that grows until equilibrium is reached. Assay time can range from 10 hours to days depending on equilibration time of the antigen and antibody. The zone diameter from the ring is linearly related to the log of protein concentration and is compared to zone diameters for known protein standards for quantification.
DNA and RNA assays
Quantitative polymerase chain reaction (qPCR)
Quantitative PCR utilizespolymerase chain reaction
The polymerase chain reaction (PCR) is a method widely used to make millions to billions of copies of a specific DNA sample rapidly, allowing scientists to amplify a very small sample of DNA (or a part of it) sufficiently to enable detailed st ...
chemistry to amplify viral DNA
Deoxyribonucleic acid (; DNA) is a polymer composed of two polynucleotide chains that coil around each other to form a double helix. The polymer carries genetic instructions for the development, functioning, growth and reproduction of al ...
or RNA
Ribonucleic acid (RNA) is a polymeric molecule that is essential for most biological functions, either by performing the function itself (non-coding RNA) or by forming a template for the production of proteins (messenger RNA). RNA and deoxyrib ...
to produce high enough concentrations for detection and quantification by fluorescence. In general, quantification by qPCR relies on serial dilutions of standards of known concentration being analyzed in parallel with the unknown samples for calibration
In measurement technology and metrology, calibration is the comparison of measurement values delivered by a device under test with those of a calibration standard of known accuracy. Such a standard could be another measurement device of known ...
and reference. Quantitative detection can be achieved using a wide variety of fluorescence detection strategies, including sequence specific probes or non-specific fluorescent dyes such as SYBR Green.
Sequence-specific probes, such as TaqMan Molecular Beacons, or Scorpion, bind only to the DNA of the appropriate sequence produced during the reaction. SYBR Green dye binds to all double-stranded DNA produced during the reaction.
While SYBR Green is easy to use, its lack of specificity and lower sensitivity lead most labs to use probe-based qPCR detection schemes. There are many variations of qPCR including the comparative threshold method, which allows relative quantification through comparison of Ct values (PCR cycles that show statistically significant increases in the product) from multiple samples that include an internal standard.
PCR amplifies all target nucleic acid
Nucleic acids are large biomolecules that are crucial in all cells and viruses. They are composed of nucleotides, which are the monomer components: a pentose, 5-carbon sugar, a phosphate group and a nitrogenous base. The two main classes of nuclei ...
, including ones originating from intact infectious viral particles, from defective viral particles as well as free nucleic acid in solution. Because of this, qPCR results (expressed in terms of genome copies/mL) are likely to be higher in quantity than TEM results. For viral quantification, the ratio of whole virions to copies of nucleic acid is seldom one to one. This is because during viral replication, the nucleic acid and viral proteins are not always produced in 1:1 ratio and viral assembly process results in complete virions as well as empty capsids and/or excess free viral genomes. In the example of foot-and-mouth disease virus, the ratio of whole virions to RNA copies within an actively replicating host cell is approximately 1:1000. Advantages of titration by qPCR include quick turnaround time (1–4 hours) and sensitivity (can detect much lower concentration of viruses than other methods).
Particle Assays
Tunable resistive pulse sensing (TRPS)
Tunable resistive pulse sensing (TRPS) is a method that allows high-throughput single particle measurements of individual virus particles, as they are driven through a size-tunable nanopore, one at a time. The technique has the advantage of simultaneously determining the size and concentration, of virus particles in solution with high resolution. This can be used in assessing sample stability and the contribution of aggregates, as well as total viral particle concentration (vp/mL). TRPS-based measurement occurs in an ionic buffer, and no pre-staining of samples is required prior to analysis, thus the technique is more rapid than those which require pre-treatment with fluorescent dyes, with a total preparation and measurement time of less than 10 minutes per sample. TRPS-bases virus analysis is commercially available through qViro-X systems, which have the ability to be decontaminated chemically by autoclaving after measurement has occurred.Single Virus Inductively Coupled Plasma Mass Spectroscopy (SV ICP-MS)
This technique is similar to Single Particle Inductively Coupled Plasma Mass Spectroscopy (SPICP-MS
Inductively coupled plasma mass spectrometry (ICP-MS) is a type of mass spectrometry that uses an inductively coupled plasma to ionize the sample. It atomizes the sample and creates atomic and small polyatomic ions, which are then detected. It ...
) discovered by Degueldre and Favarger (2003) and adapted later for other nanoparticles (e.g. gold colloids, see Degueldre et al. (2006)). The SP ICP-MS was adapted for the analysis of Single Virus Inductively Coupled Plasma Mass Spectroscopy (SV ICPMS) in a comprehensive study i.e. Degueldre (2021). This study suggests to adapting this method for single viruses (SV) identification and counting. With high resolution multi-channel sector field (MC SF) ICP-MS records in SV detection mode, the counting of master and key ions can allow analysis and identification of single viruses. The counting of 2-500 virial units can be performed in 20 s. Analyses are proposed to be carried out in Ar torch for master ion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
s: 12C+, 13C+, 14N+, 15N+, and key ions 31P+, 32S+, 33S+ and 34S+. All interferences are discussed in detail. The use of high resolution MC ICP-MS is recommended while options with anaerobic/aerobic atmospheres are explored to upgrade the analysis when using quadrupole ICP-MS. Application for two virus types (SARS-COV2 and bacteriophage T5) is investigated using time scan and fixed mass analysis for the selected virus ions allowing characterisation of the species using the N/C, P/C and S/C molar ratio's and quantification of their number concentration.
Other assays
Flow cytometry
While most flow cytometers do not have sufficient sensitivity, there are a few commercially available flow cytometers that can be used for virus quantification. A virus counter quantifies the number of intact virus particles in a sample using fluorescence to detect colocalized proteins and nucleic acids. Samples are stained with two dyes, one specific for proteins and one specific for nucleic acids, and analyzed as they flow through a laser beam. The quantity of particles producing simultaneous events on each of the two distinct fluorescence channels is determined, along with the measured sample flow rate, to calculate a concentration of virus particles (vp/mL). The results are generally similar in absolute quantity to a TEM result. The assay has a linear working range of 105–109 vp/mL and an analysis time of ~10 min with a short sample preparation time.Transmission electron microscopy (TEM)
TEM is a specialized type of microscopy that utilizes a beam of electrons focused with a magnetic field to image a sample. TEM provides imaging with 1000x greater spatial resolution than a light microscope (resolution down to 0.2 nm). An ultrathin,negatively stained In microscopy, negative staining is an established method, often used in diagnostic microscopy, for contrasting a thin specimen with an optically opaque fluid. In this technique, the background is stained, leaving the actual specimen untouched, a ...
sample is required. Sample preparations involve depositing specimens onto a coated TEM grid and negative staining with an electron-opaque liquid. Tissue embedded samples can also be examined if thinly sectioned. Sample preparations vary depending on protocol and user but generally require hours to complete. TEM images can show individual virus particles and quantitative image analysis
Image analysis or imagery analysis is the extraction of meaningful information from images; mainly from digital images by means of digital image processing techniques. Image analysis tasks can be as simple as reading barcode, bar coded tags or a ...
can be used to determine virus concentrations. These high resolution images also provide particle morphology information that most other methods cannot. Quantitative TEM results will often be greater than results from other assays as all particles, regardless of infectivity, are quantified in the reported virus-like particles per mL (vlp/mL) result. Quantitative TEM generally works well for virus concentrations greater than 106 particles/mL. Because of high instrument cost and the amount of space and support facilities needed, TEM equipment is only available in a few laboratories.
See also
* List of subviral agents * Minimal infective dose *Viral load
Viral load, also known as viral burden, is a numerical expression of the quantity of virus in a given volume of fluid, including biological and environmental specimens. It is not to be confused with viral titre or viral titer, which depends on the ...
References
{{DEFAULTSORT:Virus Quantification Laboratory techniques Immunology Virology