|
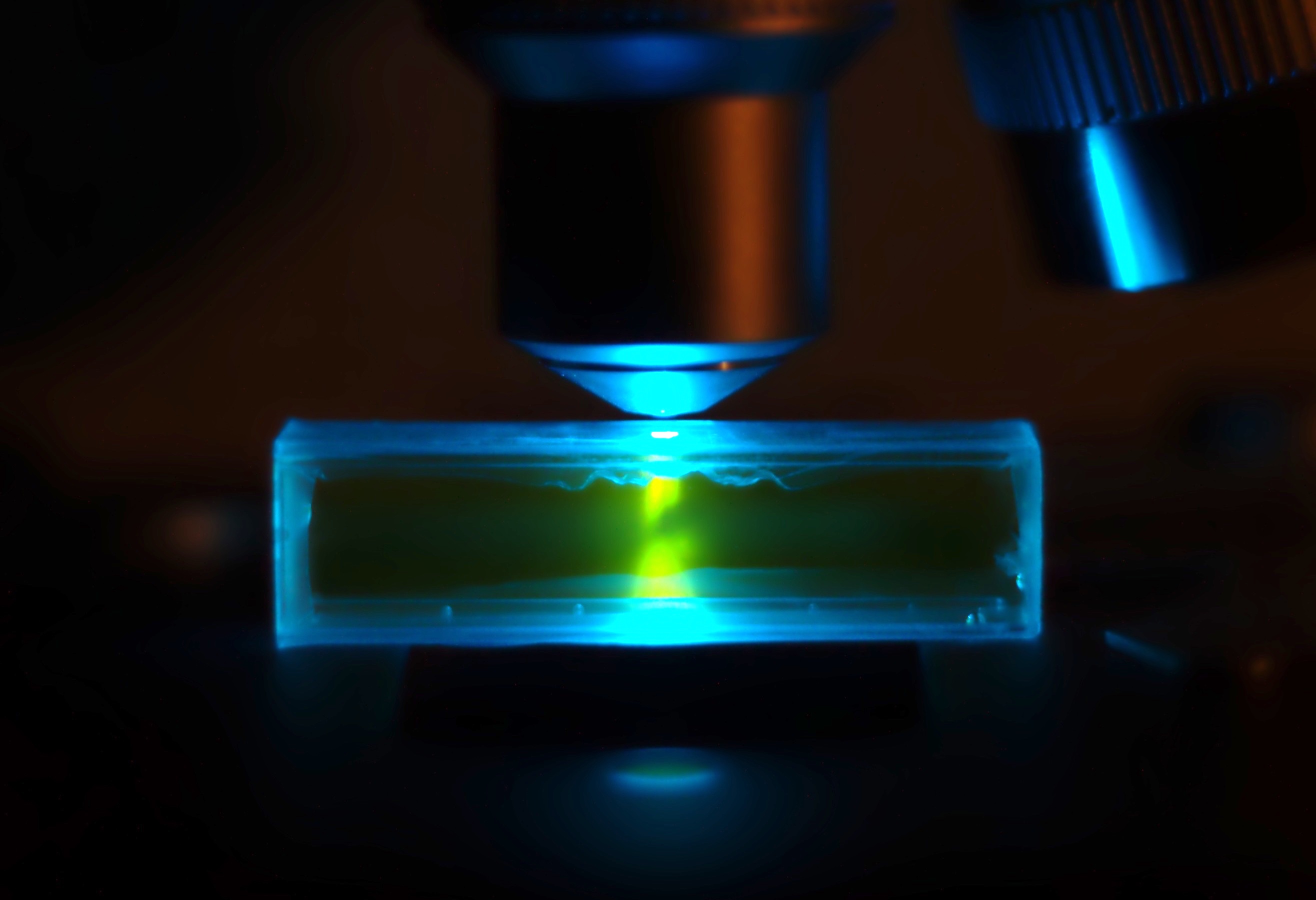
SYBR Green
SYBR Green I (SG) is an asymmetrical cyanine dye used as a nucleic acid stain in molecular biology. The SYBR family of dyes is produced by Molecular Probes Inc., now owned by Thermo Fisher Scientific. SYBR Green I binds to DNA. The resulting DNA-dye-complex best absorbs 497 nanometer blue light (λmax = 497 nm) and emits green light (λmax = 520 nm). The stain preferentially binds to double-stranded DNA, but will stain single-stranded (ss) DNA with lower performance. SYBR Green can also stain RNA with a lower performance than ssDNA. Uses SYBR Green finds usage in several areas of biochemistry Biochemistry, or biological chemistry, is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology, a ... and molecular biology. It is used as a dye for the quantification of double stranded DNA in some methods of quantitative ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gram
The gram (originally gramme; SI unit symbol g) is a Physical unit, unit of mass in the International System of Units (SI) equal to one thousandth of a kilogram. Originally defined in 1795 as "the absolute Mass versus weight, weight of a volume of pure water equal to Cube (algebra), the cube of the hundredth part of a metre [1 Cubic centimetre, cm3], and at Melting point of water, the temperature of Melting point, melting ice", the defining temperature (0 °C) was later changed to the temperature of maximum density of water (approximately 4 °C). Subsequent redefinitions agree with this original definition to within 30 Parts-per notation, parts per million (0.003%), with the maximum density of water remaining very close to 1 g/cm3, as shown by modern measurements. By the late 19th century, there was an effort to make the Base unit (measurement), base unit the kilogram and the gram a derived unit. In 1960, the new International System of Units defined a '' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
RNase
Ribonuclease (commonly abbreviated RNase) is a type of nuclease that catalyzes the degradation of RNA into smaller components. Ribonucleases can be divided into endoribonucleases and exoribonucleases, and comprise several sub-classes within the EC 2.7 (for the phosphorolytic enzymes) and 3.1 (for the hydrolytic enzymes) classes of enzymes. Function All organisms studied contain many RNases of two different classes, showing that RNA degradation is a very ancient and important process. As well as clearing of cellular RNA that is no longer required, RNases play key roles in the maturation of all RNA molecules, both messenger RNAs that carry genetic material for making proteins and non-coding RNAs that function in varied cellular processes. In addition, active RNA degradation systems are the first defense against RNA viruses and provide the underlying machinery for more advanced cellular immune strategies such as RNAi. Some cells also secrete copious quantities of non-specific RN ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyanine Dyes
Cyanines, also referred to as tetramethylindo(di)-carbocyanines are a synthetic dye family belonging to the polymethine group. Although the name derives etymologically from terms for shades of blue, the cyanine family covers the electromagnetic spectrum from near IR to UV. Chemically, cyanines are a conjugated system between two nitrogen atoms; in each resonance structure, exactly one nitrogen atom is oxidized to an iminium. Typically, they form part of a nitrogenous heterocyclic system. The main application for cyanine dyes is in biological labeling. Nevertheless, there is a wide literature on both their synthesis and uses, and cyanines are common in some CD and DVD media. Structure Cyanines have been classified in many ways: * ''Streptocyanines'' or ''open chain cyanines'': : R2N+=CH H=CH'n''-NR2 (I) * ''Hemicyanines'': : Aryl=N+=CH H=CH'n''-NR2 (II) * ''Closed chain cyanines'': :Aryl=N+=CH H=CH'n''-N=Aryl (III) Additionally, these classes are recognized: *''N ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Staining Dyes
Staining is a technique used to enhance contrast in samples, generally at the microscopic level. Stains and dyes are frequently used in histology (microscopic study of biological tissues), in cytology (microscopic study of cells), and in the medical fields of histopathology, hematology, and cytopathology that focus on the study and diagnoses of diseases at the microscopic level. Stains may be used to define biological tissues (highlighting, for example, muscle fibers or connective tissue), cell populations (classifying different blood cells), or organelles within individual cells. In biochemistry, it involves adding a class-specific (DNA, proteins, lipids, carbohydrates) dye to a substrate to qualify or quantify the presence of a specific compound. Staining and fluorescent tagging can serve similar purposes. Biological staining is also used to mark cells in flow cytometry, and to flag proteins or nucleic acids in gel electrophoresis. Light microscopes are used for viewing s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GelGreen
GelGreen is an intercalating nucleic acid stain used in molecular genetics for agarose gel DNA electrophoresis. GelGreen consists of two acridine orange subunits that are bridged by a linear oxygenated spacer. Its fluorophore, and therefore its optical properties, are essentially identical to those of other ''N''-alkylacridinium orange dyes. When exposed to ultraviolet light, it will fluoresce with a greenish color that strongly intensifies after binding to DNA. The substance is marketed as a less toxic and more sensitive alternative to ethidium bromide. GelGreen is sold as a solution in either DMSO or water. {{short description, DNA gel stain for molecular genetics See also * Ethidium bromide * GelRed * SYBR Green I * Agarose gel electrophoresis and gel electrophoresis of nucleic acids * Acridine orange Acridine orange is an organic compound that serves as a nucleic acid-selective fluorescent dye with cationic properties useful for cell cycle determination. Acridine oran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxazole Yellow
Oxazole is the parent compound for a vast class of heterocyclic aromatic organic compounds. These are azoles with an oxygen and a nitrogen separated by one carbon. Oxazoles are aromatic compounds but less so than the thiazoles. Oxazole is a weak base; its conjugate acid has a p''K''a of 0.8, compared to 7 for imidazole. Preparation The classic synthetic route the Robinson–Gabriel synthesis by dehydration of 2-acylaminoketones: The Fischer oxazole synthesis from cyanohydrins and aldehydes is also widely used: Other methods are known including the reaction of α- haloketones and formamide and the Van Leusen reaction with aldehydes and TosMIC. Biosynthesis In biomolecules, oxazoles result from the cyclization and oxidation of serine or threonine nonribosomal peptides: : Oxazoles are not as abundant in biomolecules as the related thiazoles with oxygen replaced by a sulfur atom. Reactions With a pKa of 0.8 for the conjugate acid (oxazolium salts), oxazoles are far less bas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiazole Orange
Thiazole (), or 1,3-thiazole, is a 5-membered heterocyclic compound that contains both sulfur and nitrogen. The term 'thiazole' also refers to a large family of derivatives. Thiazole itself is a pale yellow liquid with a pyridine-like odor and the molecular formula C3H3NS. The thiazole ring is notable as a component of the vitamin thiamine (B1). Molecular and electronic structure Thiazoles are members of the azoles, heterocycles that include imidazoles and oxazoles. Thiazole can also be considered a functional group when part of a larger molecule. Being planar thiazoles are characterized by significant pi-electron delocalization and have some degree of aromaticity, more so than the corresponding oxazoles. This aromaticity is evidenced by the 1H NMR chemical shift of the ring protons, which absorb between 7.27 and 8.77 ppm, indicating a strong diamagnetic ring current. The calculated pi-electron density marks C5 as the primary site for electrophilic substitution, and C2-H as s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SYBR Gold
SYBR Gold is an asymmetrical cyanine dye. It can be used as a stain for double-stranded DNA, single-stranded DNA, and RNA. SYBR Gold is the most sensitive fluorescent stain of the SYBR family of dyes for the detection of nucleic acids. The SYBR family of dyes is produced by Molecular Probes Inc., now owned by Thermo Fisher Scientific SYBR Gold is more sensitive than ethidium bromide, SYBR Green I, and SYBR Green II for detecting various types of nucleic acids. SYBR Gold's superior sensitivity is due to the high fluorescence quantum yield of the dye-nucleic acid complexes (~0.6-0.7) and the dye's large fluorescence enhancement upon binding to nucleic acids (~1000-fold). SYBR Gold can detect as little as 25 pg of DNA which makes it >10-fold more sensitive than ethidium bromide for detecting nucleic acids in denaturing urea, gyoxal, and formaldehyde gels, even with 300 nm transillumination. Fluorescence properties SYBR Gold has two fluorescence excitation maxima when bound t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SYBR Safe
SYBR Safe is a cyanine dye used as a nucleic acid stain in molecular biology. SYBR Safe is one of a number of SYBR dyes made by the Life Technologies Corporation. SYBR Safe binds to DNA. The resulting DNA-dye-complex absorbs blue light (λmax = 509 nm) and emits green light (λmax = 524 nm). A comprehensive study of Thiazole-Orange-Based DNA Dyes was published, involving SYBR Safe, SYBR Green, PicoGreen, SYTO-16, SYTO-9 and a new derivative TOPhBu. Here, the synthesis of these compounds are published and characterised spectroscopically to quantify their fluorescence enhancement upon binding to double-stranded DNA. The ability of the dyes to detect DNA at low concentrations was evaluated using two new metrics, absolute fluorescence enhancement (AFE) and relativefluorescence enhancement (RFE). They were tested in qPCR experiments showing some important differences in the sensitivity and qPCR efficiency, which facilitates the DNA marker selection for analytical purposes. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ames Test
The Ames test is a widely employed method that uses bacteria to test whether a given chemical can cause mutations in the DNA of the test organism. More formally, it is a bioassay, biological assay to assess the mutagenic potential of chemical compounds. A positive test indicates that the chemical is mutagenic and therefore may act as a carcinogen, because cancer is often linked to mutation. The test serves as a quick and convenient assay to estimate the carcinogenic potential of a compound because standard carcinogen assays on mice and rats are time-consuming (taking two to three years to complete) and expensive. However, false-positives and false-negatives are known. The procedure was described in a series of papers in the early 1970s by Bruce Ames and his group at the University of California, Berkeley. General procedure The Ames test uses several strains of the bacterium ''Salmonella typhimurium'' that carry mutations in genes involved in histidine synthesis. These strains a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carcinogen
A carcinogen () is any agent that promotes the development of cancer. Carcinogens can include synthetic chemicals, naturally occurring substances, physical agents such as ionizing and non-ionizing radiation, and biologic agents such as viruses and bacteria. Most carcinogens act by creating mutations in DNA that disrupt a cell's normal processes for regulating growth, leading to uncontrolled cellular proliferation. This occurs when the cell's DNA repair processes fail to identify DNA damage allowing the defect to be passed down to daughter cells. The damage accumulates over time. This is typically a multi-step process during which the regulatory mechanisms within the cell are gradually dismantled allowing for unchecked cellular division. The specific mechanisms for carcinogenic activity is unique to each agent and cell type. Carcinogens can be broadly categorized, however, as activation-dependent and activation-independent which relate to the agent's ability to engage dir ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |