Stepwise Reaction on:
[Wikipedia]
[Google]
[Amazon]
 A chemical reaction is a process that leads to the
A chemical reaction is a process that leads to the


 The most important elementary reactions are unimolecular and bimolecular reactions. Only one molecule is involved in a unimolecular reaction; it is transformed by isomerization or a dissociation into one or more other molecules. Such reactions require the addition of energy in the form of heat or light. A typical example of a unimolecular reaction is the cis–trans
The most important elementary reactions are unimolecular and bimolecular reactions. Only one molecule is involved in a unimolecular reaction; it is transformed by isomerization or a dissociation into one or more other molecules. Such reactions require the addition of energy in the form of heat or light. A typical example of a unimolecular reaction is the cis–trans AB -> A + B
: Dissociation of a molecule AB into fragments A and B
For A + B -> AB
Another possibility is that only a portion of one molecule is transferred to the other molecule. This type of reaction occurs, for example, in HA + B -> A + HB
for example
:NaCl + AgNO3 -> NaNO3 + AgCl(v)
2CO(g) + MoO2(s) -> 2CO2(g) + Mo(s) ;
This reaction to form CO(g) + H2O() <=> CO2(g) + H2(g)
is favored by low temperatures, but its reverse is favored by high temperatures. The shift in reaction direction tendency occurs at .
Reactions can also be characterized by their

A + B->AB
Two or more reactants yielding one product is another way to identify a synthesis reaction. One example of a synthesis reaction is the combination of 8Fe + S8->8FeS
Another example is simple hydrogen gas combined with simple oxygen gas to produce a more complex substance, such as water.To react or not to react?
Utah State Office of Education. Retrieved 4 June 2011.
AB->A + B
One example of a decomposition reaction is the 2H2O->2H2 + O2
A + BC->AC + B
One example of a single displacement reaction is when Mg + 2H2O->Mg(OH)2 (v) + H2 (^)
AB + CD->AD + CB
For example, when Pb(NO3)2 + 2KI->PbI2(v) + 2KNO3
C8H18(l) + 25/2 O2(g)->8CO2 + 9H2O(l)
releases 5500 kJ. A combustion reaction can also result from 2Mg(s) + O2->2MgO(s)
S(s) + O2(g)->SO2(g)
2Na(s) + Cl2(g)->2NaCl(s)
In the reaction, sodium metal goes from an oxidation state of 0 (a pure element) to +1: in other words, the sodium lost one electron and is said to have been oxidized. On the other hand, the chlorine gas goes from an oxidation of 0 (also a pure element) to −1: the chlorine gains one electron and is said to have been reduced. Because the chlorine is the one reduced, it is considered the electron acceptor, or in other words, induces oxidation in the sodium – thus the chlorine gas is considered the oxidizing agent. Conversely, the sodium is oxidized or is the electron donor, and thus induces a reduction in the other species and is considered the ''reducing agent''.
Which of the involved reactants would be a reducing or oxidizing agent can be predicted from the
 In complexation reactions, several
In complexation reactions, several
\underset + \underset <=> \underset + \underset
The reverse reaction is possible, and thus the acid/base and conjugated base/acid are always in equilibrium. The equilibrium is determined by the acid and base dissociation constants (''K''a and ''K''b) of the involved substances. A special case of the acid-base reaction is the neutralization where an acid and a base, taken at the exact same amounts, form a neutral

 In
In
 In
In
 In the third type of substitution reaction, radical substitution, the attacking particle is a radical. This process usually takes the form of a
In the third type of substitution reaction, radical substitution, the attacking particle is a radical. This process usually takes the form of a X. + R-H -> X-H + R.
:;R. + X2 -> R-X + X.
:: Reactions during the chain reaction of radical substitution
 The E2 mechanism also requires a base, but there the attack of the base and the elimination of the leaving group proceed simultaneously and produce no ionic intermediate. In contrast to the E1 eliminations, different stereochemical configurations are possible for the reaction product in the E2 mechanism, because the attack of the base preferentially occurs in the anti-position with respect to the leaving group. Because of the similar conditions and reagents, the E2 elimination is always in competition with the SN2-substitution.
The E2 mechanism also requires a base, but there the attack of the base and the elimination of the leaving group proceed simultaneously and produce no ionic intermediate. In contrast to the E1 eliminations, different stereochemical configurations are possible for the reaction product in the E2 mechanism, because the attack of the base preferentially occurs in the anti-position with respect to the leaving group. Because of the similar conditions and reagents, the E2 elimination is always in competition with the SN2-substitution.
 The counterpart of elimination is an addition where double or triple bonds are converted into single bonds. Similar to substitution reactions, there are several types of additions distinguished by the type of the attacking particle. For example, in the
The counterpart of elimination is an addition where double or triple bonds are converted into single bonds. Similar to substitution reactions, there are several types of additions distinguished by the type of the attacking particle. For example, in the 
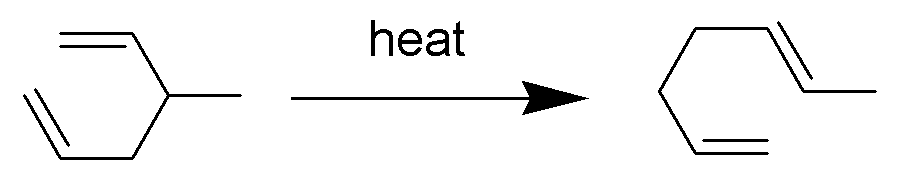 In a
In a
 Biochemical reactions are mainly controlled by complex
Biochemical reactions are mainly controlled by complex
 Chemical reactions are central to
Chemical reactions are central to
 A chemical reaction is a process that leads to the
A chemical reaction is a process that leads to the chemical
A chemical substance is a unique form of matter with constant chemical composition and characteristic properties. Chemical substances may take the form of a single element or chemical compounds. If two or more chemical substances can be combin ...
transformation of one set of chemical substance
A chemical substance is a unique form of matter with constant chemical composition and characteristic properties. Chemical substances may take the form of a single element or chemical compounds. If two or more chemical substances can be com ...
s to another. When chemical reactions occur, the atom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished fr ...
s are rearranged and the reaction is accompanied by an energy change as new products are generated. Classically, chemical
A chemical substance is a unique form of matter with constant chemical composition and characteristic properties. Chemical substances may take the form of a single element or chemical compounds. If two or more chemical substances can be combin ...
reactions encompass changes that only involve the positions of electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
s in the forming and breaking of chemical bond
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds or through the sharing of electrons a ...
s between atom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished fr ...
s, with no change to the nuclei (no change to the elements present), and can often be described by a chemical equation
A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and chemical formulas. The reactant entities are given on the left-hand side and the Product (chemistry), product entities are on the right-hand side ...
. Nuclear chemistry
Nuclear chemistry is the sub-field of chemistry dealing with radioactivity, nuclear processes, and transformations in the nuclei of atoms, such as nuclear transmutation and nuclear properties.
It is the chemistry of radioactive elements such as t ...
is a sub-discipline of chemistry that involves the chemical reactions of unstable
In dynamical systems instability means that some of the outputs or internal state (controls), states increase with time, without bounds. Not all systems that are not Stability theory, stable are unstable; systems can also be marginal stability ...
and radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is conside ...
elements where both electronic and nuclear changes can occur.
The substance (or substances) initially involved in a chemical reaction are called reactants or reagents. Chemical reactions are usually characterized by a chemical change
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an energy change as new products ...
, and they yield one or more products
Product may refer to:
Business
* Product (business), an item that can be offered to a market to satisfy the desire or need of a customer.
* Product (project management), a deliverable or set of deliverables that contribute to a business solution
...
, which usually have properties different from the reactants. Reactions often consist of a sequence of individual sub-steps, the so-called elementary reaction
An elementary reaction is a chemical reaction in which one or more chemical species react directly to form Product (chemistry), products in a single reaction step and with a single transition state. In practice, a reaction is assumed to be element ...
s, and the information on the precise course of action is part of the reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical reaction occurs.
A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage ...
. Chemical reactions are described with chemical equation
A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and chemical formulas. The reactant entities are given on the left-hand side and the Product (chemistry), product entities are on the right-hand side ...
s, which symbolically present the starting materials, end products, and sometimes intermediate products and reaction conditions.
Chemical reactions happen at a characteristic reaction rate
The reaction rate or rate of reaction is the speed at which a chemical reaction takes place, defined as proportional to the increase in the concentration of a product per unit time and to the decrease in the concentration of a reactant per u ...
at a given temperature and chemical concentration. Some reactions produce heat
In thermodynamics, heat is energy in transfer between a thermodynamic system and its surroundings by such mechanisms as thermal conduction, electromagnetic radiation, and friction, which are microscopic in nature, involving sub-atomic, ato ...
and are called exothermic reaction
In thermochemistry, an exothermic reaction is a "reaction for which the overall standard enthalpy change Δ''H''⚬ is negative." Exothermic reactions usually release heat. The term is often confused with exergonic reaction, which IUPAC define ...
s, while others may require heat to enable the reaction to occur, which are called endothermic reactions. Typically, reaction rates increase with increasing temperature because there is more thermal energy
The term "thermal energy" is often used ambiguously in physics and engineering. It can denote several different physical concepts, including:
* Internal energy: The energy contained within a body of matter or radiation, excluding the potential en ...
available to reach the activation energy necessary for breaking bonds between atoms.
A reaction may be classified as redox
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is t ...
in which oxidation
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
and reduction occur or non-redox in which there is no oxidation and reduction occurring. Most simple redox reactions may be classified as a combination, decomposition, or single displacement reaction.
Different chemical reactions are used during chemical synthesis
Chemical synthesis (chemical combination) is the artificial execution of chemical reactions to obtain one or several products. This occurs by physical and chemical manipulations usually involving one or more reactions. In modern laboratory uses ...
in order to obtain the desired product. In biochemistry
Biochemistry, or biological chemistry, is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology, a ...
, a consecutive series of chemical reactions (where the product of one reaction is the reactant of the next reaction) form metabolic pathway
In biochemistry, a metabolic pathway is a linked series of chemical reactions occurring within a cell (biology), cell. The reactants, products, and Metabolic intermediate, intermediates of an enzymatic reaction are known as metabolites, which are ...
s. These reactions are often catalyzed by protein enzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different mol ...
s. Enzymes increase the rates of biochemical reactions, so that metabolic
Metabolism (, from ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run cellular processes; the ...
syntheses and decompositions impossible under ordinary conditions can occur at the temperature and concentrations present within a cell.
The general concept of a chemical reaction has been extended to reactions between entities smaller than atoms, including nuclear reactions
In nuclear physics and nuclear chemistry, a nuclear reaction is a process in which two nuclei, or a nucleus and an external subatomic particle, collide to produce one or more new nuclides. Thus, a nuclear reaction must cause a transformation o ...
, radioactive decay
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is conside ...
s and reactions between elementary particle
In particle physics, an elementary particle or fundamental particle is a subatomic particle that is not composed of other particles. The Standard Model presently recognizes seventeen distinct particles—twelve fermions and five bosons. As a c ...
s, as described by quantum field theory
In theoretical physics, quantum field theory (QFT) is a theoretical framework that combines Field theory (physics), field theory and the principle of relativity with ideas behind quantum mechanics. QFT is used in particle physics to construct phy ...
.
History
Chemical reactions such as combustion in fire,fermentation
Fermentation is a type of anaerobic metabolism which harnesses the redox potential of the reactants to make adenosine triphosphate (ATP) and organic end products. Organic molecules, such as glucose or other sugars, are catabolized and reduce ...
and the reduction of ores to metals were known since antiquity. Initial theories of transformation of materials were developed by Greek philosophers, such as the Four-Element Theory of Empedocles
Empedocles (; ; , 444–443 BC) was a Ancient Greece, Greek pre-Socratic philosopher and a native citizen of Akragas, a Greek city in Sicily. Empedocles' philosophy is known best for originating the Cosmogony, cosmogonic theory of the four cla ...
stating that any substance is composed of the four basic elements – fire, water, air and earth. In the Middle Ages
In the history of Europe, the Middle Ages or medieval period lasted approximately from the 5th to the late 15th centuries, similarly to the post-classical period of global history. It began with the fall of the Western Roman Empire and ...
, chemical transformations were studied by alchemist
Alchemy (from the Arabic word , ) is an ancient branch of natural philosophy, a philosophical and protoscientific tradition that was historically practised in China, India, the Muslim world, and Europe. In its Western form, alchemy is first ...
s. They attempted, in particular, to convert lead
Lead () is a chemical element; it has Chemical symbol, symbol Pb (from Latin ) and atomic number 82. It is a Heavy metal (elements), heavy metal that is density, denser than most common materials. Lead is Mohs scale, soft and Ductility, malleabl ...
into gold
Gold is a chemical element; it has chemical symbol Au (from Latin ) and atomic number 79. In its pure form, it is a brightness, bright, slightly orange-yellow, dense, soft, malleable, and ductile metal. Chemically, gold is a transition metal ...
, for which purpose they used reactions of lead and lead-copper alloys with sulfur
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms ...
.
The artificial production of chemical substances already was a central goal for medieval alchemists. Examples include the synthesis of ammonium chloride
Ammonium chloride is an inorganic chemical compound with the chemical formula , also written as . It is an ammonium salt of hydrogen chloride. It consists of ammonium cations and chloride anions . It is a white crystalline salt (chemistry), sal ...
from organic substances as described in the works (c. 850–950) attributed to Jābir ibn Ḥayyān, or the production of mineral acids
A mineral acid (or inorganic acid) is an acid derived from one or more inorganic compounds, as opposed to organic acids which are acidic, organic compounds. All mineral acids form hydrogen ions and the conjugate base when dissolved in water.
Cha ...
such as sulfuric and nitric acid
Nitric acid is an inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into nitrogen oxide, oxides of nitrogen. Most com ...
s by later alchemists, starting from c. 1300. The production of mineral acids involved the heating of sulfate and nitrate minerals such as copper sulfate Copper sulfate may refer to:
* Copper(II) sulfate, CuSO4, a common, greenish blue compound used as a fungicide and herbicide
* Copper(I) sulfate, Cu2SO4, an unstable white solid which is uncommonly used
{{chemistry index
Copper compounds ...
, alum
An alum () is a type of chemical compound, usually a hydrated double salt, double sulfate salt (chemistry), salt of aluminium with the general chemical formula, formula , such that is a valence (chemistry), monovalent cation such as potassium ...
and saltpeter
Potassium nitrate is a chemical compound with a sharp, salty, bitter taste and the chemical formula . It is a potassium salt of nitric acid. This salt consists of potassium cations and nitrate anions , and is therefore an alkali metal nitrate ...
. In the 17th century, Johann Rudolph Glauber produced hydrochloric acid
Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungency, pungent smell. It is classified as a acid strength, strong acid. It is ...
and sodium sulfate
Sodium sulfate (also known as sodium sulphate or sulfate of soda) is the inorganic compound with formula Na2SO4 as well as several related hydrates. All forms are white solids that are highly soluble in water. With an annual production of 6 mill ...
by reacting sulfuric acid and sodium chloride
Sodium chloride , commonly known as Salt#Edible salt, edible salt, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. It is transparent or translucent, brittle, hygroscopic, and occurs a ...
. With the development of the lead chamber process in 1746 and the Leblanc process
The Leblanc process was an early industrial process for making ''soda ash'' ( sodium carbonate) used throughout the 19th century, named after its inventor, Nicolas Leblanc. It involved two stages: making sodium sulfate from sodium chloride, fol ...
, allowing large-scale production of sulfuric acid and sodium carbonate
Sodium carbonate (also known as washing soda, soda ash, sal soda, and soda crystals) is the inorganic compound with the formula and its various hydrates. All forms are white, odourless, water-soluble salts that yield alkaline solutions in water ...
, respectively, chemical reactions became implemented into the industry. Further optimization of sulfuric acid technology resulted in the contact process
The contact process is a method of producing sulfuric acid in the high concentrations needed for industrial processes. Platinum was originally used as the catalyst for this reaction; however, because it is susceptible to reacting with arsenic impu ...
in the 1880s, and the Haber process
The Haber process, also called the Haber–Bosch process, is the main industrial procedure for the ammonia production, production of ammonia. It converts atmospheric nitrogen (N2) to ammonia (NH3) by a reaction with hydrogen (H2) using finely di ...
was developed in 1909–1910 for ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
synthesis.
From the 16th century, researchers including Jan Baptist van Helmont
Jan Baptist van Helmont ( , ; 12 January 1580 – 30 December 1644) was a chemist, physiologist, and physician from Brussels. He worked during the years just after Paracelsus and the rise of iatrochemistry, and is sometimes considered to be ...
, Robert Boyle
Robert Boyle (; 25 January 1627 – 31 December 1691) was an Anglo-Irish natural philosopher, chemist, physicist, Alchemy, alchemist and inventor. Boyle is largely regarded today as the first modern chemist, and therefore one of the foun ...
, and Isaac Newton
Sir Isaac Newton () was an English polymath active as a mathematician, physicist, astronomer, alchemist, theologian, and author. Newton was a key figure in the Scientific Revolution and the Age of Enlightenment, Enlightenment that followed ...
tried to establish theories of experimentally observed chemical transformations. The phlogiston theory
The phlogiston theory, a superseded scientific theory, postulated the existence of a fire-like element dubbed phlogiston () contained within combustible bodies and released during combustion. The name comes from the Ancient Greek (''burnin ...
was proposed in 1667 by Johann Joachim Becher. It postulated the existence of a fire-like element called "phlogiston", which was contained within combustible bodies and released during combustion
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel (the reductant) and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion ...
. This proved to be false in 1785 by Antoine Lavoisier
Antoine-Laurent de Lavoisier ( ; ; 26 August 17438 May 1794), When reduced without charcoal, it gave off an air which supported respiration and combustion in an enhanced way. He concluded that this was just a pure form of common air and that i ...
who found the correct explanation of the combustion as a reaction with oxygen from the air.
Joseph Louis Gay-Lussac
Joseph Louis Gay-Lussac ( , ; ; 6 December 1778 – 9 May 1850) was a French chemist and physicist. He is known mostly for his discovery that water is made of two parts hydrogen and one part oxygen by volume (with Alexander von Humboldt), f ...
recognized in 1808 that gases always react in a certain relationship with each other. Based on this idea and the atomic theory of John Dalton
John Dalton (; 5 or 6 September 1766 – 27 July 1844) was an English chemist, physicist and meteorologist. He introduced the atomic theory into chemistry. He also researched Color blindness, colour blindness; as a result, the umbrella term ...
, Joseph Proust
Joseph Louis Proust (26 September 1754 – 5 July 1826) was a French people, French chemist. He was best known for his discovery of the law of definite proportions in 1797, stating that chemical compounds always combine in constant proportions.
...
had developed the law of definite proportions
In chemistry, the law of definite proportions, sometimes called Proust's law or the law of constant composition, states that a given
chemical compound contains its constituent elements in a fixed ratio (by mass) and does not depend on its source ...
, which later resulted in the concepts of stoichiometry
Stoichiometry () is the relationships between the masses of reactants and Product (chemistry), products before, during, and following chemical reactions.
Stoichiometry is based on the law of conservation of mass; the total mass of reactants must ...
and chemical equation
A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and chemical formulas. The reactant entities are given on the left-hand side and the Product (chemistry), product entities are on the right-hand side ...
s.
Regarding the organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
, it was long believed that compounds obtained from living organisms were too complex to be obtained synthetically. According to the concept of vitalism
Vitalism is a belief that starts from the premise that "living organisms are fundamentally different from non-living entities because they contain some non-physical element or are governed by different principles than are inanimate things." Wher ...
, organic matter was endowed with a "vital force" and distinguished from inorganic materials. This separation was ended however by the synthesis of urea
Urea, also called carbamide (because it is a diamide of carbonic acid), is an organic compound with chemical formula . This amide has two Amine, amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest am ...
from inorganic precursors by Friedrich Wöhler
Friedrich Wöhler Royal Society of London, FRS(For) HonFRSE (; 31 July 180023 September 1882) was a German chemist known for his work in both organic chemistry, organic and inorganic chemistry, being the first to isolate the chemical elements be ...
in 1828. Other chemists who brought major contributions to organic chemistry include Alexander William Williamson
Alexander William Williamson Fellow of the Royal Society, FRS FRSE Chemical Society, PCS MRIA (1 May 18246 May 1904) was an English chemist. He is best known today for the Williamson ether synthesis.
Life
Williamson was born in 1824 in Wands ...
with his synthesis
Synthesis or synthesize may refer to:
Science Chemistry and biochemistry
*Chemical synthesis, the execution of chemical reactions to form a more complex molecule from chemical precursors
**Organic synthesis, the chemical synthesis of organi ...
of ether
In organic chemistry, ethers are a class of compounds that contain an ether group, a single oxygen atom bonded to two separate carbon atoms, each part of an organyl group (e.g., alkyl or aryl). They have the general formula , where R and R� ...
s and Christopher Kelk Ingold
Sir Christopher Kelk Ingold (28 October 1893 – 8 December 1970) was a British chemist based in Leeds and London. His groundbreaking work in the 1920s and 1930s on reaction mechanisms and the electronic structure of organic compounds was resp ...
, who, among many discoveries, established the mechanisms of substitution reaction
A substitution reaction (also known as single displacement reaction or single substitution reaction) is a chemical reaction during which one functional group in a chemical compound is replaced by another functional group. Substitution reactions ar ...
s.
Characteristics
The general characteristics of chemical reactions are: * Evolution of a gas * Formation of aprecipitate
In an aqueous solution, precipitation is the "sedimentation of a solid material (a precipitate) from a liquid solution". The solid formed is called the precipitate. In case of an inorganic chemical reaction leading to precipitation, the chemic ...
* Change in temperature
Temperature is a physical quantity that quantitatively expresses the attribute of hotness or coldness. Temperature is measurement, measured with a thermometer. It reflects the average kinetic energy of the vibrating and colliding atoms making ...
* Change in state
State most commonly refers to:
* State (polity), a centralized political organization that regulates law and society within a territory
**Sovereign state, a sovereign polity in international law, commonly referred to as a country
**Nation state, a ...
Equations

Chemical equation
A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and chemical formulas. The reactant entities are given on the left-hand side and the Product (chemistry), product entities are on the right-hand side ...
s are used to graphically illustrate chemical reactions. They consist of chemical
A chemical substance is a unique form of matter with constant chemical composition and characteristic properties. Chemical substances may take the form of a single element or chemical compounds. If two or more chemical substances can be combin ...
or structural formula
The structural formula of a chemical compound is a graphic representation of the molecular structure (determined by structural chemistry methods), showing how the atoms are connected to one another. The chemical bonding within the molecule is al ...
s of the reactants on the left and those of the products on the right. They are separated by an arrow (→) which indicates the direction and type of the reaction; the arrow is read as the word "yields". The tip of the arrow points in the direction in which the reaction proceeds. A double arrow () pointing in opposite directions is used for equilibrium reactions. Equations should be balanced according to the stoichiometry
Stoichiometry () is the relationships between the masses of reactants and Product (chemistry), products before, during, and following chemical reactions.
Stoichiometry is based on the law of conservation of mass; the total mass of reactants must ...
, the number of atoms of each species should be the same on both sides of the equation. This is achieved by scaling the number of involved molecules (A, B, C and D in a schematic example below) by the appropriate integers ''a, b, c'' and ''d''.
:
More elaborate reactions are represented by reaction schemes, which in addition to starting materials and products show important intermediates or transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked w ...
s. Also, some relatively minor additions to the reaction can be indicated above the reaction arrow; examples of such additions are water, heat, illumination, a catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
, etc. Similarly, some minor products can be placed below the arrow, often with a minus sign.
Retrosynthetic analysis
Retrosynthetic analysis is a technique for solving problems in the planning of organic syntheses. This is achieved by transforming a target molecule into simpler precursor structures regardless of any potential reactivity/interaction with reagents. ...
can be applied to design a complex synthesis reaction. Here the analysis starts from the products, for example by splitting selected chemical bonds, to arrive at plausible initial reagents. A special arrow (⇒) is used in retro reactions.
Elementary reactions
Theelementary reaction
An elementary reaction is a chemical reaction in which one or more chemical species react directly to form Product (chemistry), products in a single reaction step and with a single transition state. In practice, a reaction is assumed to be element ...
is the smallest division into which a chemical reaction can be decomposed, it has no intermediate products. Most experimentally observed reactions are built up from many elementary reactions that occur in parallel or sequentially. The actual sequence of the individual elementary reactions is known as reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical reaction occurs.
A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage ...
. An elementary reaction involves a few molecules, usually one or two, because of the low probability for several molecules to meet at a certain time.
isomerization
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomer ...
, in which the cis-form of a compound converts to the trans-form or vice versa.
In a typical dissociation reaction, a bond in a molecule splits (ruptures) resulting in two molecular fragments. The splitting can be homolytic or heterolytic. In the first case, the bond is divided so that each product retains an electron and becomes a neutral radical. In the second case, both electrons of the chemical bond remain with one of the products, resulting in charged ion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
s. Dissociation plays an important role in triggering chain reaction
A chain reaction is a sequence of reactions where a reactive product or by-product causes additional reactions to take place. In a chain reaction, positive feedback leads to a self-amplifying chain of events.
Chain reactions are one way that sys ...
s, such as hydrogen–oxygen or polymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many fo ...
reactions.
:bimolecular
In chemistry, molecularity is the number of molecules that come together to react in an elementary reaction, elementary (single-step) reactionAtkins, P.; de Paula, J. Physical Chemistry. Oxford University Press, 2014 and is equal to the sum of Sto ...
reactions, two molecules collide and react with each other. Their merger is called chemical synthesis
Chemical synthesis (chemical combination) is the artificial execution of chemical reactions to obtain one or several products. This occurs by physical and chemical manipulations usually involving one or more reactions. In modern laboratory uses ...
or an addition reaction
In organic chemistry, an addition reaction is an organic reaction in which two or more molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, ...
.
:redox
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is t ...
and acid-base reactions. In redox reactions, the transferred particle is an electron, whereas in acid-base reactions it is a proton. This type of reaction is also called metathesis.
:Chemical equilibrium
Most chemical reactions are reversible; that is, they can and do run in both directions. The forward and reverse reactions are competing with each other and differ in reaction rates. These rates depend on the concentration and therefore change with the time of the reaction: the reverse rate gradually increases and becomes equal to the rate of the forward reaction, establishing the so-called chemical equilibrium. The time to reach equilibrium depends on parameters such as temperature, pressure, and the materials involved, and is determined by the minimum free energy. In equilibrium, theGibbs free energy
In thermodynamics, the Gibbs free energy (or Gibbs energy as the recommended name; symbol is a thermodynamic potential that can be used to calculate the maximum amount of Work (thermodynamics), work, other than Work (thermodynamics)#Pressure–v ...
of reaction must be zero. The pressure dependence can be explained with the Le Chatelier's principle
In chemistry, Le Chatelier's principle (pronounced or ) is a principle used to predict the effect of a change in conditions on chemical equilibrium. Other names include Chatelier's principle, Braun–Le Chatelier principle, Le Chatelier–Braun p ...
. For example, an increase in pressure due to decreasing volume causes the reaction to shift to the side with fewer moles of gas.
The reaction yield stabilizes at equilibrium but can be increased by removing the product from the reaction mixture or changed by increasing the temperature or pressure. A change in the concentrations of the reactants does not affect the equilibrium constant but does affect the equilibrium position.
Thermodynamics
Chemical reactions are determined by the laws ofthermodynamics
Thermodynamics is a branch of physics that deals with heat, Work (thermodynamics), work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed b ...
. Reactions can proceed by themselves if they are exergonic
An exergonic process is one which there is a positive flow of energy from the system to the surroundings. This is in contrast with an endergonic process. Constant pressure, constant temperature reactions are exergonic if and only if the Gibbs ...
, that is if they release free energy. The associated free energy change of the reaction is composed of the changes of two different thermodynamic quantities, enthalpy
Enthalpy () is the sum of a thermodynamic system's internal energy and the product of its pressure and volume. It is a state function in thermodynamics used in many measurements in chemical, biological, and physical systems at a constant extern ...
and entropy
Entropy is a scientific concept, most commonly associated with states of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynamics, where it was first recognized, to the micros ...
:
:; .
:: : free energy, : enthalpy, : temperature, : entropy, : difference (change between original and product)
Reactions can be exothermic
In thermodynamics, an exothermic process () is a thermodynamic process or reaction that releases energy from the system to its surroundings, usually in the form of heat, but also in a form of light (e.g. a spark, flame, or flash), electricity (e ...
, where Δ''H'' is negative and energy is released. Typical examples of exothermic reactions are combustion
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel (the reductant) and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion ...
, precipitation
In meteorology, precipitation is any product of the condensation of atmospheric water vapor that falls from clouds due to gravitational pull. The main forms of precipitation include drizzle, rain, rain and snow mixed ("sleet" in Commonwe ...
and crystallization
Crystallization is a process that leads to solids with highly organized Atom, atoms or Molecule, molecules, i.e. a crystal. The ordered nature of a crystalline solid can be contrasted with amorphous solids in which atoms or molecules lack regu ...
, in which ordered solids are formed from disordered gaseous or liquid phases. In contrast, in endothermic
An endothermic process is a chemical or physical process that absorbs heat from its surroundings. In terms of thermodynamics, it is a thermodynamic process with an increase in the enthalpy (or internal energy ) of the system.Oxtoby, D. W; Gillis, ...
reactions, heat is consumed from the environment. This can occur by increasing the entropy of the system, often through the formation of gaseous or dissolved reaction products, which have higher entropy. Since the entropy term in the free-energy change increases with temperature, many endothermic reactions preferably take place at high temperatures. On the contrary, many exothermic reactions such as crystallization occur preferably at lower temperatures. A change in temperature can sometimes reverse the sign of the enthalpy of a reaction, as for the carbon monoxide
Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
reduction of molybdenum dioxide:
:carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
and molybdenum
Molybdenum is a chemical element; it has Symbol (chemistry), symbol Mo (from Neo-Latin ''molybdaenum'') and atomic number 42. The name derived from Ancient Greek ', meaning lead, since its ores were confused with lead ores. Molybdenum minerals hav ...
is endothermic at low temperatures, becoming less so with increasing temperature. Δ''H''° is zero at , and the reaction becomes exothermic above that temperature.
Changes in temperature can also reverse the direction tendency of a reaction. For example, the water gas shift reaction
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known living organisms ( ...
:internal energy
The internal energy of a thermodynamic system is the energy of the system as a state function, measured as the quantity of energy necessary to bring the system from its standard internal state to its present internal state of interest, accoun ...
change, which takes into account changes in the entropy, volume and chemical potential
In thermodynamics, the chemical potential of a Chemical specie, species is the energy that can be absorbed or released due to a change of the particle number of the given species, e.g. in a chemical reaction or phase transition. The chemical potent ...
s. The latter depends, among other things, on the activities of the involved substances.
:;
:: : internal energy, : entropy, : pressure, : chemical potential, : number of molecules, : small change sign
Kinetics
The speed at which reactions take place is studied byreaction kinetics
Chemical kinetics, also known as reaction kinetics, is the branch of physical chemistry that is concerned with understanding the rates of chemical reactions. It is different from chemical thermodynamics, which deals with the direction in which a ...
. The rate depends on various parameters, such as:
* Reactant concentrations, which usually make the reaction happen at a faster rate if raised through increased collisions per unit of time. Some reactions, however, have rates that are ''independent'' of reactant concentrations, due to a limited number of catalytic sites. These are called zero order reactions.
* Surface area
The surface area (symbol ''A'') of a solid object is a measure of the total area that the surface of the object occupies. The mathematical definition of surface area in the presence of curved surfaces is considerably more involved than the d ...
available for contact between the reactants, in particular solid ones in heterogeneous systems. Larger surface areas lead to higher reaction rates.
* Pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country and eve ...
– increasing the pressure decreases the volume between molecules and therefore increases the frequency of collisions between the molecules.
* Activation energy
In the Arrhenius model of reaction rates, activation energy is the minimum amount of energy that must be available to reactants for a chemical reaction to occur. The activation energy (''E''a) of a reaction is measured in kilojoules per mole (k ...
, which is defined as the amount of energy required to make the reaction start and carry on spontaneously. Higher activation energy implies that the reactants need more energy to start than a reaction with lower activation energy.
* Temperature
Temperature is a physical quantity that quantitatively expresses the attribute of hotness or coldness. Temperature is measurement, measured with a thermometer. It reflects the average kinetic energy of the vibrating and colliding atoms making ...
, which hastens reactions if raised, since higher temperature increases the energy of the molecules, creating more collisions per unit of time,
* The presence or absence of a catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
. Catalysts are substances that make weak bonds with reactants or intermediates and change the pathway (mechanism) of a reaction which in turn increases the speed of a reaction by lowering the activation energy
In the Arrhenius model of reaction rates, activation energy is the minimum amount of energy that must be available to reactants for a chemical reaction to occur. The activation energy (''E''a) of a reaction is measured in kilojoules per mole (k ...
needed for the reaction to take place. A catalyst is not destroyed or changed during a reaction, so it can be used again.
* For some reactions, the presence of electromagnetic radiation
In physics, electromagnetic radiation (EMR) is a self-propagating wave of the electromagnetic field that carries momentum and radiant energy through space. It encompasses a broad spectrum, classified by frequency or its inverse, wavelength ...
, most notably ultraviolet light
Ultraviolet radiation, also known as simply UV, is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight and constitutes about 10% of th ...
, is needed to promote the breaking of bonds to start the reaction. This is particularly true for reactions involving radicals
Radical (from Latin: ', root) may refer to:
Politics and ideology Politics
*Classical radicalism, the Radical Movement that began in late 18th century Britain and spread to continental Europe and Latin America in the 19th century
*Radical politics ...
.
Several theories allow calculating the reaction rates at the molecular level. This field is referred to as reaction dynamics. The rate ''v'' of a first-order reaction, which could be the disintegration of a substance A, is given by:
:
Its integration yields:
:
Here ''k'' is the first-order rate constant, having dimension 1/time, ''t'') is the concentration at a time ''t'' and sub>0 is the initial concentration. The rate of a first-order reaction depends only on the concentration and the properties of the involved substance, and the reaction itself can be described with a characteristic half-life Half-life is a mathematical and scientific description of exponential or gradual decay.
Half-life, half life or halflife may also refer to:
Film
* Half-Life (film), ''Half-Life'' (film), a 2008 independent film by Jennifer Phang
* ''Half Life: ...
. More than one time constant is needed when describing reactions of higher order. The temperature dependence of the rate constant usually follows the Arrhenius equation
In physical chemistry, the Arrhenius equation is a formula for the temperature dependence of reaction rates. The equation was proposed by Svante Arrhenius in 1889, based on the work of Dutch chemist Jacobus Henricus van 't Hoff who had noted in 188 ...
:
:
where ''E''a is the activation energy and ''k''B is the Boltzmann constant
The Boltzmann constant ( or ) is the proportionality factor that relates the average relative thermal energy of particles in a ideal gas, gas with the thermodynamic temperature of the gas. It occurs in the definitions of the kelvin (K) and the ...
. One of the simplest models of reaction rate is the collision theory
Collision theory is a principle of chemistry used to predict the rates of chemical reactions. It states that when suitable particles of the Reagent, reactant hit each other with the correct orientation, only a certain amount of collisions result ...
. More realistic models are tailored to a specific problem and include the transition state theory
In chemistry, transition state theory (TST) explains the reaction rates of elementary chemical reactions. The theory assumes a special type of chemical equilibrium (quasi-equilibrium) between reactants and activated transition state complexes.
...
, the calculation of the potential energy surface
A potential energy surface (PES) or energy landscape describes the energy of a Physical system, system, especially a collection of atoms, in terms of certain Parameter, parameters, normally the positions of the atoms. The Surface (mathematics), ...
, the Marcus theory and the Rice–Ramsperger–Kassel–Marcus (RRKM) theory.
Reaction types
Four basic types
Synthesis
In a synthesis reaction, two or more simple substances combine to form a more complex substance. These reactions are in the general form:iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
and sulfur
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms ...
to form iron(II) sulfide
Iron(II) sulfide or ferrous sulfide (Br.E. sulphide) is one of a family of chemical compounds and minerals with the approximate chemical formula, formula . Iron sulfides are often iron-deficient non-stoichiometric. All are black, water-insoluble ...
:
Utah State Office of Education. Retrieved 4 June 2011.
Decomposition
A decomposition reaction is when a more complex substance breaks down into its more simple parts. It is thus the opposite of a synthesis reaction and can be written aselectrolysis
In chemistry and manufacturing, electrolysis is a technique that uses Direct current, direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of c ...
of water to make oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
and hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
gas:
Single displacement
In a single displacement reaction, a single uncombined element replaces another in a compound; in other words, one element trades places with another element in a compound. These reactions come in the general form of:magnesium
Magnesium is a chemical element; it has Symbol (chemistry), symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 ...
replaces hydrogen in water to make solid magnesium hydroxide
Magnesium hydroxide is an inorganic compound with the chemical formula Mg(OH)2. It occurs in nature as the mineral brucite. It is a white solid with low solubility in water (). Magnesium hydroxide is a common component of antacids, such as milk o ...
and hydrogen gas:
Double displacement
In a double displacement reaction, the anions and cations of two compounds switch places and form two entirely different compounds. These reactions are in the general form:barium chloride
Barium chloride is an inorganic compound with the formula . It is one of the most common water-soluble salts of barium. Like most other water-soluble barium salts, it is a white powder, highly toxic, and imparts a yellow-green coloration to a flam ...
(BaCl2) and magnesium sulfate
Magnesium sulfate or magnesium sulphate is a chemical compound, a salt with the formula , consisting of magnesium cations (20.19% by mass) and sulfate anions . It is a white crystalline solid, soluble in water but not in ethanol.
Magnesi ...
(MgSO4) react, the SO42− anion switches places with the 2Cl− anion, giving the compounds BaSO4 and MgCl2.
Another example of a double displacement reaction is the reaction of lead(II) nitrate with potassium iodide
Potassium iodide is a chemical compound, medication, and dietary supplement. It is a medication used for treating hyperthyroidism, in radiation emergencies, and for protecting the thyroid gland when certain types of radiopharmaceuticals are u ...
to form lead(II) iodide
Lead(II) iodide (or lead iodide) is a chemical compound with the formula . At room temperature, it is a bright yellow odorless crystalline solid, that becomes orange and red when heated. It was formerly called plumbous iodide.
The compound curre ...
and potassium nitrate
Potassium nitrate is a chemical compound with a sharp, salty, bitter taste and the chemical formula . It is a potassium salt of nitric acid. This salt consists of potassium cations and nitrate anions , and is therefore an alkali metal nit ...
:
Forward and backward reactions
According toLe Chatelier's Principle
In chemistry, Le Chatelier's principle (pronounced or ) is a principle used to predict the effect of a change in conditions on chemical equilibrium. Other names include Chatelier's principle, Braun–Le Chatelier principle, Le Chatelier–Braun p ...
, reactions may proceed in the forward or reverse direction until they end or reach equilibrium
Equilibrium may refer to:
Film and television
* ''Equilibrium'' (film), a 2002 science fiction film
* '' The Story of Three Loves'', also known as ''Equilibrium'', a 1953 romantic anthology film
* "Equilibrium" (''seaQuest 2032'')
* ''Equilibr ...
.
Forward reactions
Reactions that proceed in the forward direction (from left to right) to approach equilibrium are often called spontaneous reactions, that is, is negative, which means that if they occur at constant temperature and pressure, they decrease theGibbs free energy
In thermodynamics, the Gibbs free energy (or Gibbs energy as the recommended name; symbol is a thermodynamic potential that can be used to calculate the maximum amount of Work (thermodynamics), work, other than Work (thermodynamics)#Pressure–v ...
of the reaction. They require less energy to proceed in the forward direction. Reactions are usually written as forward reactions in the direction in which they are spontaneous. Examples:
* Reaction of hydrogen and oxygen to form water.
: +
* Dissociation of acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main compone ...
in water into acetate
An acetate is a salt formed by the combination of acetic acid with a base (e.g. alkaline, earthy, metallic, nonmetallic, or radical base). "Acetate" also describes the conjugate base or ion (specifically, the negatively charged ion called ...
ions and hydronium ion
In chemistry, hydronium (hydroxonium in traditional British English) is the cation , also written as , the type of oxonium ion produced by protonation of water. It is often viewed as the positive ion present when an Arrhenius acid is dissolved in ...
s.
: + +
Backward reactions
Reactions that proceed in the backward direction to approach equilibrium are often called non-spontaneous reactions, that is, is positive, which means that if they occur at constant temperature and pressure, they increase theGibbs free energy
In thermodynamics, the Gibbs free energy (or Gibbs energy as the recommended name; symbol is a thermodynamic potential that can be used to calculate the maximum amount of Work (thermodynamics), work, other than Work (thermodynamics)#Pressure–v ...
of the reaction. They require input of energy to proceed in the forward direction. Examples include:
* Charging a normal DC battery (consisting of electrolytic cell
An electrolytic cell is an electrochemical cell that utilizes an external source of electrical energy to force a chemical reaction that would otherwise not occur. The external energy source is a voltage applied between the cell's two electrodes; ...
s) from an external electrical power source
* Photosynthesis
Photosynthesis ( ) is a system of biological processes by which photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical energy necessary to fuel their metabo ...
driven by absorption of electromagnetic radiation
In physics, electromagnetic radiation (EMR) is a self-propagating wave of the electromagnetic field that carries momentum and radiant energy through space. It encompasses a broad spectrum, classified by frequency or its inverse, wavelength ...
usually in the form of sunlight
: + + → +
Combustion
In acombustion
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel (the reductant) and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion ...
reaction, an element or compound reacts with an oxidant, usually oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
, often producing energy in the form of heat
In thermodynamics, heat is energy in transfer between a thermodynamic system and its surroundings by such mechanisms as thermal conduction, electromagnetic radiation, and friction, which are microscopic in nature, involving sub-atomic, ato ...
or light
Light, visible light, or visible radiation is electromagnetic radiation that can be visual perception, perceived by the human eye. Visible light spans the visible spectrum and is usually defined as having wavelengths in the range of 400– ...
. Combustion reactions frequently involve a hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and Hydrophobe, hydrophobic; their odor is usually fain ...
. For instance, the combustion of 1 mole (114 g) of octane in oxygen
carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
, magnesium
Magnesium is a chemical element; it has Symbol (chemistry), symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 ...
or sulfur
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms ...
reacting with oxygen.
Oxidation and reduction
Redox
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is t ...
reactions can be understood in terms of the transfer of electrons from one involved species (reducing agent
In chemistry, a reducing agent (also known as a reductant, reducer, or electron donor) is a chemical species that "donates" an electron to an (called the , , , or ).
Examples of substances that are common reducing agents include hydrogen, carbon ...
) to another (oxidizing agent
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or " accepts"/"receives" an electron from a (called the , , or ''electron donor''). In ot ...
). In this process, the former species is ''oxidized'' and the latter is ''reduced''. Though sufficient for many purposes, these descriptions are not precisely correct. Oxidation is better defined as an increase in oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons ...
of atoms and reduction as a decrease in oxidation state. In practice, the transfer of electrons will always change the oxidation state, but there are many reactions that are classed as "redox" even though no electron transfer occurs (such as those involving covalent
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
bonds).
In the following redox reaction, hazardous sodium
Sodium is a chemical element; it has Symbol (chemistry), symbol Na (from Neo-Latin ) and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 element, group 1 of the peri ...
metal reacts with toxic chlorine
Chlorine is a chemical element; it has Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between ...
gas to form the ionic compound sodium chloride
Sodium chloride , commonly known as Salt#Edible salt, edible salt, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. It is transparent or translucent, brittle, hygroscopic, and occurs a ...
, or common table salt:
electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
of their elements. Elements with low electronegativities, such as most metals, easily donate electrons and oxidize – they are reducing agents. On the contrary, many oxides or ions with high oxidation numbers of their non-oxygen atoms, such as , , , , or , can gain one or two extra electrons and are strong oxidizing agents.
For some main-group element
In chemistry and atomic physics, the main group is the group (periodic table), group of chemical element, elements (sometimes called the representative elements) whose lightest members are represented by helium, lithium, beryllium, boron, carbon ...
s the number of electrons donated or accepted in a redox reaction can be predicted from the electron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon ato ...
of the reactant element. Elements try to reach the low-energy noble gas
The noble gases (historically the inert gases, sometimes referred to as aerogens) are the members of Group (periodic table), group 18 of the periodic table: helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), radon (Rn) and, in some ...
configuration, and therefore alkali metals
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names ...
and halogens
The halogens () are a group (periodic table), group in the periodic table consisting of six chemically related chemical element, elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and the radioactive elements astatine (At) and ten ...
will donate and accept one electron, respectively. Noble gases themselves are chemically inactive.
The overall redox reaction can be balanced by combining the oxidation and reduction half-reactions multiplied by coefficients such that the number of electrons lost in the oxidation equals the number of electrons gained in the reduction.
An important class of redox reactions are the electrolytic electrochemical
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference and identifiable chemical change. These reactions involve electrons moving via an electronically conducting phase (typi ...
reactions, where electrons from the power supply at the negative electrode are used as the reducing agent and electron withdrawal at the positive electrode as the oxidizing agent. These reactions are particularly important for the production of chemical elements, such as chlorine
Chlorine is a chemical element; it has Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between ...
or aluminium
Aluminium (or aluminum in North American English) is a chemical element; it has chemical symbol, symbol Al and atomic number 13. It has a density lower than that of other common metals, about one-third that of steel. Aluminium has ...
. The reverse process, in which electrons are released in redox reactions and chemical energy
Chemical energy is the energy of chemical substances that is released when the substances undergo a chemical reaction and transform into other substances. Some examples of storage media of chemical energy include batteries, Schmidt-Rohr, K. (20 ...
is converted to electrical energy
Electrical energy is the energy transferred as electric charges move between points with different electric potential, that is, as they move across a voltage, potential difference. As electric potential is lost or gained, work is done changing the ...
, is possible and used in batteries.
Complexation
 In complexation reactions, several
In complexation reactions, several ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
s react with a metal atom to form a coordination complex
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of chemical bond, bound molecules or ions, that are in turn known as ' ...
. This is achieved by providing lone pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC ''Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone ...
s of the ligand into empty orbitals of the metal atom and forming dipolar bonds. The ligands are Lewis base
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
s, they can be both ions and neutral molecules, such as carbon monoxide, ammonia or water. The number of ligands that react with a central metal atom can be found using the 18-electron rule, saying that the valence shell
In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a chemical bond if the outermost shell is not closed. In a single covalent bond, a shared pair forms with b ...
s of a transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. The lanthanide and actinid ...
will collectively accommodate 18 electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
s, whereas the symmetry of the resulting complex can be predicted with the crystal field theory and ligand field theory. Complexation reactions also include ligand exchange, in which one or more ligands are replaced by another, and redox processes which change the oxidation state of the central metal atom.
Acid–base reactions
In theBrønsted–Lowry acid–base theory
The Brønsted–Lowry theory (also called proton theory of acids and bases) is an acid–base reaction theory which was developed independently in 1923 by physical chemists Johannes Nicolaus Brønsted (in Denmark) and Thomas Martin Lowry (in ...
, an acid–base reaction
In chemistry, an acid–base reaction is a chemical reaction that occurs between an acid and a base. It can be used to determine pH via titration. Several theoretical frameworks provide alternative conceptions of the reaction mechanisms an ...
involves a transfer of proton
A proton is a stable subatomic particle, symbol , Hydron (chemistry), H+, or 1H+ with a positive electric charge of +1 ''e'' (elementary charge). Its mass is slightly less than the mass of a neutron and approximately times the mass of an e ...
s (H+) from one species (the acid
An acid is a molecule or ion capable of either donating a proton (i.e. Hydron, hydrogen cation, H+), known as a Brønsted–Lowry acid–base theory, Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis ...
) to another (the base). When a proton is removed from an acid, the resulting species is termed that acid's conjugate base
A conjugate acid, within the Brønsted–Lowry acid–base theory, is a chemical compound formed when an acid gives a proton () to a base—in other words, it is a base with a hydrogen ion added to it, as it loses a hydrogen ion in the reve ...
. When the proton is accepted by a base, the resulting species is termed that base's conjugate acid
A conjugate acid, within the Brønsted–Lowry acid–base theory, is a chemical compound formed when an acid gives a proton () to a base—in other words, it is a base with a hydrogen ion added to it, as it loses a hydrogen ion in the rever ...
. In other words, acids act as proton donors and bases act as proton acceptors according to the following equation:
salt
In common usage, salt is a mineral composed primarily of sodium chloride (NaCl). When used in food, especially in granulated form, it is more formally called table salt. In the form of a natural crystalline mineral, salt is also known as r ...
.
Acid-base reactions can have different definitions depending on the acid-base concept employed. Some of the most common are:
* Arrhenius definition: Acids dissociate in water releasing H3O+ ions; bases dissociate in water releasing OH− ions.
* Brønsted–Lowry definition: Acids are proton (H+) donors, bases are proton acceptors; this includes the Arrhenius definition.
* Lewis definition: Acids are electron-pair acceptors, and bases are electron-pair donors; this includes the Brønsted-Lowry definition.
Precipitation
Precipitation
In meteorology, precipitation is any product of the condensation of atmospheric water vapor that falls from clouds due to gravitational pull. The main forms of precipitation include drizzle, rain, rain and snow mixed ("sleet" in Commonwe ...
is the formation of a solid in a solution or inside another solid during a chemical reaction. It usually takes place when the concentration of dissolved ions exceeds the solubility
In chemistry, solubility is the ability of a chemical substance, substance, the solute, to form a solution (chemistry), solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form su ...
limit and forms an insoluble salt. This process can be assisted by adding a precipitating agent or by the removal of the solvent. Rapid precipitation results in an amorphous
In condensed matter physics and materials science, an amorphous solid (or non-crystalline solid) is a solid that lacks the long-range order that is a characteristic of a crystal. The terms "glass" and "glassy solid" are sometimes used synonymousl ...
or microcrystalline residue and a slow process can yield single crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macros ...
s. The latter can also be obtained by recrystallization from microcrystalline salts.
Solid-state reactions
Reactions can take place between two solids. However, because of the relatively smalldiffusion
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical p ...
rates in solids, the corresponding chemical reactions are very slow in comparison to liquid and gas phase reactions. They are accelerated by increasing the reaction temperature and finely dividing the reactant to increase the contacting surface area.
Reactions at the solid/gas interface
The reaction can take place at the solid, gas interface, surfaces at very low pressure such asultra-high vacuum
Ultra-high vacuum (often spelled ultrahigh in American English, UHV) is the vacuum regime characterised by pressures lower than about . UHV conditions are created by pumping the gas out of a UHV chamber. At these low pressures the mean free path of ...
. Via scanning tunneling microscopy
A scanning tunneling microscope (STM) is a type of scanning probe microscope used for imaging surfaces at the atomic level. Its development in 1981 earned its inventors, Gerd Binnig and Heinrich Rohrer, then at IBM Zürich, the Nobel Prize in ...
, it is possible to observe reactions at the solid, gas interface in real space, if the time scale of the reaction is in the correct range. Reactions at the solid, gas interface are in some cases related to catalysis.
Photochemical reactions
photochemical reactions Organic photochemistry encompasses organic reactions that are induced by the action of light. The absorption of ultraviolet light by organic molecules often leads to reactions. In the earliest days, sunlight was employed, while in more modern times ...
, atoms and molecules absorb energy (photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless particles that can ...
s) of the illumination light and convert it into an excited state
In quantum mechanics
Quantum mechanics is the fundamental physical Scientific theory, theory that describes the behavior of matter and of light; its unusual characteristics typically occur at and below the scale of atoms. Reprinted, Add ...
. They can then release this energy by breaking chemical bonds, thereby producing radicals. Photochemical reactions include hydrogen–oxygen reactions, radical polymerization, chain reaction
A chain reaction is a sequence of reactions where a reactive product or by-product causes additional reactions to take place. In a chain reaction, positive feedback leads to a self-amplifying chain of events.
Chain reactions are one way that sys ...
s and rearrangement reaction
In organic chemistry, a rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another at ...
s.
Many important processes involve photochemistry. The premier example is photosynthesis
Photosynthesis ( ) is a system of biological processes by which photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical energy necessary to fuel their metabo ...
, in which most plants use solar energy
Solar energy is the radiant energy from the Sun's sunlight, light and heat, which can be harnessed using a range of technologies such as solar electricity, solar thermal energy (including solar water heating) and solar architecture. It is a ...
to convert carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
and water into glucose
Glucose is a sugar with the Chemical formula#Molecular formula, molecular formula , which is often abbreviated as Glc. It is overall the most abundant monosaccharide, a subcategory of carbohydrates. It is mainly made by plants and most algae d ...
, disposing of oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
as a side-product. Humans rely on photochemistry for the formation of vitamin D, and vision
Vision, Visions, or The Vision may refer to:
Perception Optical perception
* Visual perception, the sense of sight
* Visual system, the physical mechanism of eyesight
* Computer vision, a field dealing with how computers can be made to gain und ...
is initiated by a photochemical reaction of rhodopsin
Rhodopsin, also known as visual purple, is a protein encoded by the ''RHO'' gene and a G-protein-coupled receptor (GPCR). It is a light-sensitive receptor protein that triggers visual phototransduction in rod cells. Rhodopsin mediates dim ...
. In fireflies
The Lampyridae are a family of elateroid beetles with more than 2,000 described species, many of which are light-emitting. They are soft-bodied beetles commonly called fireflies, lightning bugs, or glowworms for their conspicuous production ...
, an enzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different mol ...
in the abdomen catalyzes a reaction that results in bioluminescence
Bioluminescence is the emission of light during a chemiluminescence reaction by living organisms. Bioluminescence occurs in multifarious organisms ranging from marine vertebrates and invertebrates, as well as in some Fungus, fungi, microorgani ...
. Many significant photochemical reactions, such as ozone formation, occur in the Earth atmosphere and constitute atmospheric chemistry
Atmospheric chemistry is a branch of atmospheric science that studies the chemistry of the Earth's atmosphere and that of other planets. This multidisciplinary approach of research draws on environmental chemistry, physics, meteorology, comput ...
.
Catalysis
catalysis
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
, the reaction does not proceed directly, but through a reaction with a third substance known as catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
. Although the catalyst takes part in the reaction, forming weak bonds with reactants or intermediates, it is returned to its original state by the end of the reaction and so is not consumed. However, it can be inhibited, deactivated or destroyed by secondary processes. Catalysts can be used in a different phase (heterogeneous
Homogeneity and heterogeneity are concepts relating to the uniformity of a substance, process or image. A homogeneous feature is uniform in composition or character (i.e., color, shape, size, weight, height, distribution, texture, language, i ...
) or in the same phase (homogeneous
Homogeneity and heterogeneity are concepts relating to the uniformity of a substance, process or image. A homogeneous feature is uniform in composition or character (i.e., color, shape, size, weight, height, distribution, texture, language, i ...
) as the reactants. In heterogeneous catalysis, typical secondary processes include coking
Coking is the process of heating coal in the absence of oxygen to a temperature above to drive off the volatile components of the raw coal, leaving behind a hard, strong, porous material with a high carbon content called coke. Coke is predomina ...
where the catalyst becomes covered by polymer
A polymer () is a chemical substance, substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeat unit, repeating subunits derived from one or more species of monomers. Due to their br ...
ic side products. Additionally, heterogeneous catalysts can dissolve into the solution in a solid-liquid system or evaporate in a solid–gas system. Catalysts can only speed up the reaction – chemicals that slow down the reaction are called inhibitors. Substances that increase the activity of catalysts are called promoters, and substances that deactivate catalysts are called catalytic poisons. With a catalyst, a reaction that is kinetically inhibited by high activation energy can take place in the circumvention of this activation energy.
Heterogeneous catalysts are usually solids, powdered in order to maximize their surface area. Of particular importance in heterogeneous catalysis are the platinum group
The platinum-group metals (PGMs) are six noble, precious metallic elements clustered together in the periodic table. These elements are all transition metals in the d-block (groups 8, 9, and 10, periods 5 and 6).
The six platinum-group ...
metals and other transition metals, which are used in hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated ...
s, catalytic reforming
Catalytic reforming is a chemical process used to convert petroleum naphtha, naphthas from crude oil into liquid products called reformates, which are premium "blending stocks" for high-octane gasoline. The process converts low-octane linear hydr ...
and in the synthesis of commodity chemicals such as nitric acid
Nitric acid is an inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into nitrogen oxide, oxides of nitrogen. Most com ...
and ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
. Acids are an example of a homogeneous catalyst, they increase the nucleophilicity of carbonyl
In organic chemistry, a carbonyl group is a functional group with the formula , composed of a carbon atom double bond, double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds (such a ...
s, allowing a reaction that would not otherwise proceed with electrophiles. The advantage of homogeneous catalysts is the ease of mixing them with the reactants, but they may also be difficult to separate from the products. Therefore, heterogeneous catalysts are preferred in many industrial processes.
Reactions in organic chemistry
Inorganic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
, in addition to oxidation, reduction or acid-base reactions, a number of other reactions can take place which involves covalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
s between carbon atoms or carbon and heteroatom
In chemistry, a heteroatom () is, strictly, any atom that is not carbon or hydrogen.
Organic chemistry
In practice, the term is mainly used more specifically to indicate that non-carbon atoms have replaced carbon in the backbone of the molecular ...
s (such as oxygen, nitrogen, halogen
The halogens () are a group in the periodic table consisting of six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and the radioactive elements astatine (At) and tennessine (Ts), though some authors would ...
s, etc.). Many specific reactions in organic chemistry are name reactions designated after their discoverers.
One of the most industrially important reactions is the cracking of heavy hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and Hydrophobe, hydrophobic; their odor is usually fain ...
s at oil refineries
An oil refinery or petroleum refinery is an industrial process plant where petroleum (crude oil) is transformed and refined into products such as gasoline (petrol), diesel fuel, asphalt base, fuel oils, heating oil, kerosene, liquefied pet ...
to create smaller, simpler molecules. This process is used to manufacture gasoline
Gasoline ( North American English) or petrol ( Commonwealth English) is a petrochemical product characterized as a transparent, yellowish, and flammable liquid normally used as a fuel for spark-ignited internal combustion engines. When for ...
. Specific types of organic reactions may be grouped by their reaction mechanisms (particularly substitution, addition and elimination) or by the types of products they produce (for example, methylation
Methylation, in the chemistry, chemical sciences, is the addition of a methyl group on a substrate (chemistry), substrate, or the substitution of an atom (or group) by a methyl group. Methylation is a form of alkylation, with a methyl group replac ...
, polymerisation
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many form ...
and halogenation
In chemistry, halogenation is a chemical reaction which introduces one or more halogens into a chemical compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymers, drug ...
).
Substitution
In asubstitution reaction
A substitution reaction (also known as single displacement reaction or single substitution reaction) is a chemical reaction during which one functional group in a chemical compound is replaced by another functional group. Substitution reactions ar ...
, a functional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
in a particular chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
is replaced by another group. These reactions can be distinguished by the type of substituting species into a nucleophilic, electrophilic
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carr ...
or radical substitution.
In the first type, a nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
, an atom or molecule with an excess of electrons and thus a negative charge or partial charge
In atomic physics, a partial charge (or net atomic charge) is a non-integer charge value when measured in elementary charge units. It is represented by the Greek lowercase delta (𝛿), namely 𝛿− or 𝛿+.
Partial charges are created due to ...
, replaces another atom or part of the "substrate
Substrate may refer to:
Physical layers
*Substrate (biology), the natural environment in which an organism lives, or the surface or medium on which an organism grows or is attached
** Substrate (aquatic environment), the earthy material that exi ...
" molecule. The electron pair from the nucleophile attacks the substrate forming a new bond, while the leaving group
In organic chemistry, a leaving group typically means a Chemical species, molecular fragment that departs with an electron, electron pair during a reaction step with heterolysis (chemistry), heterolytic bond cleavage. In this usage, a ''leaving gr ...
departs with an electron pair. The nucleophile may be electrically neutral or negatively charged, whereas the substrate is typically neutral or positively charged. Examples of nucleophiles are hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. It ...
ion, alkoxide
In chemistry, an alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They are written as , where R is the organyl substituent. Alkoxides are strong bases and, whe ...
s, amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
s and halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fl ...
s. This type of reaction is found mainly in aliphatic hydrocarbons, and rarely in aromatic hydrocarbon
Aromatic compounds or arenes are organic compounds "with a chemistry typified by benzene" and "cyclically conjugated."
The word "aromatic" originates from the past grouping of molecules based on odor, before their general chemical properties were ...
. The latter have high electron density and enter nucleophilic aromatic substitution
A nucleophilic aromatic substitution (SNAr) is a substitution reaction in organic chemistry in which the nucleophile displaces a good leaving group, such as a halide, on an aromatic ring. Aromatic rings are usually nucleophilic, but some aromatic c ...
only with very strong electron withdrawing groups. Nucleophilic substitution can take place by two different mechanisms, SN1 and SN2. In their names, S stands for substitution, N for nucleophilic, and the number represents the kinetic order of the reaction, unimolecular or bimolecular.
The SN1 reaction proceeds in two steps. First, the leaving group
In organic chemistry, a leaving group typically means a Chemical species, molecular fragment that departs with an electron, electron pair during a reaction step with heterolysis (chemistry), heterolytic bond cleavage. In this usage, a ''leaving gr ...
is eliminated creating a carbocation
Carbocation is a general term for ions with a positively charged carbon atom. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further ...
. This is followed by a rapid reaction with the nucleophile.
In the SN2 mechanisms, the nucleophile forms a transition state with the attacked molecule, and only then the leaving group is cleaved. These two mechanisms differ in the stereochemistry
Stereochemistry, a subdiscipline of chemistry, studies the spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereoisomers, which are defined ...
of the products. SN1 leads to the non-stereospecific addition and does not result in a chiral center, but rather in a set of geometric isomers (''cis/trans''). In contrast, a reversal ( Walden inversion) of the previously existing stereochemistry is observed in the SN2 mechanism.
Electrophilic substitution is the counterpart of the nucleophilic substitution in that the attacking atom or molecule, an electrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively Electric charge, charged, have an ...
, has low electron density and thus a positive charge. Typical electrophiles are the carbon atom of carbonyl group
In organic chemistry, a carbonyl group is a functional group with the formula , composed of a carbon atom double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds (such as aldehydes ...
s, carbocations or sulfur
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms ...
or nitronium
The nitronium ion, , is a cation. It is an onium ion because its nitrogen atom has +1 charge, similar to ammonium ion . It is created by the removal of an electron from the paramagnetic nitrogen dioxide molecule
A molecule is a grou ...
cations. This reaction takes place almost exclusively in aromatic hydrocarbons, where it is called electrophilic aromatic substitution
Electrophilic aromatic substitution (SEAr) is an organic reaction in which an atom that is attached to an aromatic ring, aromatic system (usually hydrogen) is replaced by an electrophile. Some of the most important electrophilic aromatic substitut ...
. The electrophile attack results in the so-called σ-complex, a transition state in which the aromatic system is abolished. Then, the leaving group, usually a proton, is split off and the aromaticity is restored. An alternative to aromatic substitution is electrophilic aliphatic substitution. It is similar to the nucleophilic aliphatic substitution and also has two major types, SE1 and SE2.
chain reaction
A chain reaction is a sequence of reactions where a reactive product or by-product causes additional reactions to take place. In a chain reaction, positive feedback leads to a self-amplifying chain of events.
Chain reactions are one way that sys ...
, for example in the reaction of alkanes with halogens. In the first step, light or heat disintegrates the halogen-containing molecules producing radicals. Then the reaction proceeds as an avalanche until two radicals meet and recombine.
:;Addition and elimination
Theaddition
Addition (usually signified by the Plus and minus signs#Plus sign, plus symbol, +) is one of the four basic Operation (mathematics), operations of arithmetic, the other three being subtraction, multiplication, and Division (mathematics), divis ...
and its counterpart, the elimination, are reactions that change the number of substituents on the carbon atom, and form or cleave multiple bonds. Double
Double, The Double or Dubble may refer to:
Mathematics and computing
* Multiplication by 2
* Double precision, a floating-point representation of numbers that is typically 64 bits in length
* A double number of the form x+yj, where j^2=+1
* A ...
and triple bond
A triple bond in chemistry is a chemical bond between two atoms involving six Electron pair bond, bonding electrons instead of the usual two in a covalent bond, covalent single bond. Triple bonds are stronger than the equivalent covalent bond, sin ...
s can be produced by eliminating a suitable leaving group. Similar to the nucleophilic substitution, there are several possible reaction mechanisms that are named after the respective reaction order. In the E1 mechanism, the leaving group is ejected first, forming a carbocation. The next step, the formation of the double bond, takes place with the elimination of a proton (deprotonation
Deprotonation (or dehydronation) is the removal (transfer) of a proton (or hydron, or hydrogen cation), (H+) from a Brønsted–Lowry acid in an acid–base reaction.Henry Jakubowski, Biochemistry Online Chapter 2A3, https://employees.csbsju.ed ...
). The leaving order is reversed in the E1cb mechanism, that is the proton is split off first. This mechanism requires the participation of a base. Because of the similar conditions, both reactions in the E1 or E1cb elimination always compete with the SN1 substitution.
electrophilic addition
In organic chemistry, an electrophilic addition (AE) reaction is an addition reaction where a chemical compound containing a double or triple bond has a π bond broken, with the formation of two new σ bonds.March, Jerry; (1985). Advanced Organic ...
of hydrogen bromide
Hydrogen bromide is the inorganic compound with the formula . It is a hydrogen halide consisting of hydrogen and bromine. A colorless gas, it dissolves in water, forming hydrobromic acid, which is saturated at 68.85% HBr by weight at room temper ...
, an electrophile (proton) attacks the double bond forming a carbocation
Carbocation is a general term for ions with a positively charged carbon atom. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further ...
, which then reacts with the nucleophile (bromine). The carbocation can be formed on either side of the double bond depending on the groups attached to its ends, and the preferred configuration can be predicted with the Markovnikov's rule
In organic chemistry, Markovnikov's rule or Markownikoff's rule describes the outcome of some addition reactions. The rule was formulated by Russian chemist Vladimir Markovnikov in 1870.
Explanation
The rule states that with the addition of a ...
. This rule states that "In the heterolytic addition of a polar molecule to an alkene or alkyne, the more electronegative (nucleophilic) atom (or part) of the polar molecule becomes attached to the carbon atom bearing the smaller number of hydrogen atoms."
If the addition of a functional group takes place at the less substituted carbon atom of the double bond, then the electrophilic substitution with acids is not possible. In this case, one has to use the hydroboration–oxidation reaction, wherein the first step, the boron
Boron is a chemical element; it has symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it has three ...
atom acts as electrophile and adds to the less substituted carbon atom. In the second step, the nucleophilic hydroperoxide
Hydroperoxides or peroxols are Chemical compound, compounds of the form ROOH, where R stands for any group, typically Organic compound, organic, which contain the hydroperoxy functional group (). Hydroperoxide also refers to the hydroperoxide anio ...
or halogen anion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
attacks the boron atom.
While the addition to the electron-rich alkenes and alkynes is mainly electrophilic, the nucleophilic addition
In organic chemistry, a nucleophilic addition (AN) reaction is an addition reaction where a chemical compound with an electrophilic double or triple bond reacts with a nucleophile, such that the double or triple bond is broken. Nucleophilic addit ...
plays an important role in the carbon-heteroatom multiple bonds, and especially its most important representative, the carbonyl group. This process is often associated with elimination so that after the reaction the carbonyl group is present again. It is, therefore, called an addition-elimination reaction and may occur in carboxylic acid derivatives such as chlorides, esters or anhydrides. This reaction is often catalyzed by acids or bases, where the acids increase the electrophilicity of the carbonyl group by binding to the oxygen atom, whereas the bases enhance the nucleophilicity of the attacking nucleophile.
Nucleophilic addition
In organic chemistry, a nucleophilic addition (AN) reaction is an addition reaction where a chemical compound with an electrophilic double or triple bond reacts with a nucleophile, such that the double or triple bond is broken. Nucleophilic addit ...
of a carbanion
In organic chemistry, a carbanion is an anion with a lone pair attached to a tervalent carbon atom. This gives the carbon atom a negative charge.
Formally, a carbanion is the conjugate base of a carbon acid:
:
where B stands for the base (chemist ...
or another nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
to the double bond of an alpha, beta-unsaturated carbonyl compound can proceed via the Michael reaction, which belongs to the larger class of conjugate additions. This is one of the most useful methods for the mild formation of C–C bonds.
Some additions which can not be executed with nucleophiles and electrophiles can be succeeded with free radicals. As with the free-radical substitution, the radical addition
In organic chemistry, free-radical addition is an addition reaction which involves free radicals. These reactions can happen due to the free radicals having an unpaired electron in their valence shell, making them highly reactive. Radical addi ...
proceeds as a chain reaction, and such reactions are the basis of the free-radical polymerization.
Other organic reaction mechanisms
 In a
In a rearrangement reaction
In organic chemistry, a rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another at ...
, the carbon skeleton of a molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
is rearranged to give a structural isomer
In chemistry, a structural isomer (or constitutional isomer in the IUPAC nomenclature) of a compound is a compound that contains the same number and type of atoms, but with a different connectivity (i.e. arrangement of bonds) between them. The ...
of the original molecule. These include hydride shift reactions such as the Wagner-Meerwein rearrangement, where a hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
, alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl group is derived from a cy ...
or aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used ...
group migrates from one carbon to a neighboring carbon. Most rearrangements are associated with the breaking and formation of new carbon-carbon bonds. Other examples are sigmatropic reaction such as the Cope rearrangement.
Cyclic rearrangements include cycloaddition
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more Unsaturated hydrocarbon, unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of th ...
s and, more generally, pericyclic reactions, wherein two or more double bond-containing molecules form a cyclic molecule. An important example of cycloaddition reaction is the Diels–Alder reaction
In organic chemistry, the Diels–Alder reaction is a chemical reaction between a Conjugated system, conjugated diene and a substituted alkene, commonly termed the Diels–Alder reaction#The dienophile, dienophile, to form a substituted cyclohexe ...
(the so-called +2cycloaddition) between a conjugated diene
In organic chemistry, a diene ( ); also diolefin, ) or alkadiene) is a covalent compound that contains two double bonds, usually among carbon atoms. They thus contain two alk''ene'' units, with the standard prefix ''di'' of systematic nome ...
and a substituted alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
to form a substituted cyclohexene
Cyclohexene is a hydrocarbon with the formula . It is a cycloalkene. At room temperature, cyclohexene is a colorless liquid with a sharp odor. Among its uses, it is an chemical intermediate, intermediate in the commercial synthesis of nylon.
Prod ...
system.
Whether a certain cycloaddition would proceed depends on the electronic orbitals of the participating species, as only orbitals with the same sign of wave function
In quantum physics, a wave function (or wavefunction) is a mathematical description of the quantum state of an isolated quantum system. The most common symbols for a wave function are the Greek letters and (lower-case and capital psi (letter) ...
will overlap and interact constructively to form new bonds. Cycloaddition is usually assisted by light or heat. These perturbations result in a different arrangement of electrons in the excited state of the involved molecules and therefore in different effects. For example, the +2Diels-Alder reactions can be assisted by heat whereas the +2cycloaddition is selectively induced by light. Because of the orbital character, the potential for developing stereoisomeric products upon cycloaddition is limited, as described by the Woodward–Hoffmann rules
The Woodward–Hoffmann rules (or the pericyclic selection rules) are a set of rules devised by Robert Burns Woodward and Roald Hoffmann to rationalize or predict certain aspects of the stereochemistry and activation energy of Pericyclic reaction, ...
.
Biochemical reactions
protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
s called enzymes
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as pro ...
, which are usually specialized to catalyze
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
only a single, specific reaction. The reaction takes place in the active site
In biology and biochemistry, the active site is the region of an enzyme where substrate molecules bind and undergo a chemical reaction. The active site consists of amino acid residues that form temporary bonds with the substrate, the ''binding s ...
, a small part of the enzyme which is usually found in a cleft or pocket lined by amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 a ...
residues, and the rest of the enzyme is used mainly for stabilization. The catalytic action of enzymes relies on several mechanisms including the molecular shape ("induced fit"), bond strain, proximity and orientation of molecules relative to the enzyme, proton donation or withdrawal (acid/base catalysis), electrostatic interactions and many others.
The biochemical reactions that occur in living organisms are collectively known as metabolism
Metabolism (, from ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run cellular processes; the co ...
. Among the most important of its mechanisms is the anabolism
Anabolism () is the set of metabolic pathways that construct macromolecules like DNA or RNA from smaller units. These reactions require energy, known also as an Endergonic reaction, endergonic process. Anabolism is the building-up aspect of metabo ...
, in which different DNA
Deoxyribonucleic acid (; DNA) is a polymer composed of two polynucleotide chains that coil around each other to form a double helix. The polymer carries genetic instructions for the development, functioning, growth and reproduction of al ...
and enzyme-controlled processes result in the production of large molecules such as protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
s and carbohydrates
A carbohydrate () is a biomolecule composed of carbon (C), hydrogen (H), and oxygen (O) atoms. The typical hydrogen-to-oxygen atomic ratio is 2:1, analogous to that of water, and is represented by the empirical formula (where ''m'' and ''n'' ma ...
from smaller units. Bioenergetics
Bioenergetics is a field in biochemistry and cell biology that concerns energy flow through living systems. This is an active area of biological research that includes the study of the transformation of energy in living organisms and the study o ...
studies the sources of energy for such reactions. Important energy sources are glucose
Glucose is a sugar with the Chemical formula#Molecular formula, molecular formula , which is often abbreviated as Glc. It is overall the most abundant monosaccharide, a subcategory of carbohydrates. It is mainly made by plants and most algae d ...
and oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
, which can be produced by plants via photosynthesis
Photosynthesis ( ) is a system of biological processes by which photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical energy necessary to fuel their metabo ...
or assimilated from food and air, respectively. All organisms use this energy to produce adenosine triphosphate
Adenosine triphosphate (ATP) is a nucleoside triphosphate that provides energy to drive and support many processes in living cell (biology), cells, such as muscle contraction, nerve impulse propagation, and chemical synthesis. Found in all known ...
(ATP), which can then be used to energize other reactions. Decomposition of organic material by fungi
A fungus (: fungi , , , or ; or funguses) is any member of the group of eukaryotic organisms that includes microorganisms such as yeasts and mold (fungus), molds, as well as the more familiar mushrooms. These organisms are classified as one ...
, bacteria
Bacteria (; : bacterium) are ubiquitous, mostly free-living organisms often consisting of one Cell (biology), biological cell. They constitute a large domain (biology), domain of Prokaryote, prokaryotic microorganisms. Typically a few micr ...
and other micro-organisms is also within the scope of biochemistry
Biochemistry, or biological chemistry, is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology, a ...
.
Applications
 Chemical reactions are central to
Chemical reactions are central to chemical engineering
Chemical engineering is an engineering field which deals with the study of the operation and design of chemical plants as well as methods of improving production. Chemical engineers develop economical commercial processes to convert raw materials ...
, where they are used for the synthesis of new compounds from natural raw materials such as petroleum
Petroleum, also known as crude oil or simply oil, is a naturally occurring, yellowish-black liquid chemical mixture found in geological formations, consisting mainly of hydrocarbons. The term ''petroleum'' refers both to naturally occurring un ...
, mineral ore
Ore is natural rock or sediment that contains one or more valuable minerals, typically including metals, concentrated above background levels, and that is economically viable to mine and process. The grade of ore refers to the concentration ...
s, and oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
in air. It is essential to make the reaction as efficient as possible, maximizing the yield and minimizing the number of reagents, energy inputs and waste. Catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
s are especially helpful for reducing the energy required for the reaction and increasing its reaction rate
The reaction rate or rate of reaction is the speed at which a chemical reaction takes place, defined as proportional to the increase in the concentration of a product per unit time and to the decrease in the concentration of a reactant per u ...
.
Some specific reactions have their niche applications. For example, the thermite
Thermite () is a pyrotechnic composition of powder metallurgy, metal powder and metal oxide. When ignited by heat or chemical reaction, thermite undergoes an exothermic redox, reduction-oxidation (redox) reaction. Most varieties are not explos ...
reaction is used to generate light and heat in pyrotechnics
Pyrotechnics is the science and craft of creating fireworks, but also includes safety matches, oxygen candles, Pyrotechnic fastener, explosive bolts (and other fasteners), parts of automotive airbags, as well as gas-pressure blasting in mining, q ...
and welding
Welding is a fabrication (metal), fabrication process that joins materials, usually metals or thermoplastics, primarily by using high temperature to melting, melt the parts together and allow them to cool, causing Fusion welding, fusion. Co ...
. Although it is less controllable than the more conventional oxy-fuel welding
Principle of burn cutting
Oxy-fuel welding (commonly called oxyacetylene welding, oxy welding, or gas welding in the United States) and oxy-fuel cutting are processes that use fuel gases (or liquid fuels such as gasoline or petrol, diesel, bio ...
, arc welding
Arc welding is a welding process that is used to join metal to metal by using electricity to create enough heat to melt metal, and the melted metals, when cool, result in a joining of the metals. It is a type of welding that uses a welding power ...
and flash welding
Flash welding is a type of resistance welding that does not use any filler metals. The pieces of metal to be welded are set apart at a predetermined distance based on material thickness, material composition, and desired Material properties, prope ...
, it requires much less equipment and is still used to mend rails, especially in remote areas.
Monitoring
Mechanisms of monitoring chemical reactions depend strongly on the reaction rate. Relatively slow processes can be analyzed in situ for the concentrations and identities of the individual ingredients. Important tools of real-time analysis are the measurement of pH and analysis of optical absorption (color) and emission spectra. A less accessible but rather efficient method is the introduction of a radioactive isotope into the reaction and monitoring how it changes over time and where it moves to; this method is often used to analyze the redistribution of substances in the human body. Faster reactions are usually studied withultrafast laser spectroscopy
Ultrafast laser spectroscopy is a category of spectroscopic techniques using ultrashort pulse lasers for the study of dynamics on extremely short time scales (attoseconds to nanoseconds). Different methods are used to examine the dynamics of cha ...
where utilization of femtosecond
A femtosecond is a unit of time in the International System of Units (SI) equal to 10 or of a second; that is, one quadrillionth, or one millionth of one billionth, of a second.
A femtosecond is to a second, as a second is to approximately 31.6 ...
laser
A laser is a device that emits light through a process of optical amplification based on the stimulated emission of electromagnetic radiation. The word ''laser'' originated as an acronym for light amplification by stimulated emission of radi ...
s allows short-lived transition states to be monitored at a time scaled down to a few femtoseconds. Atkins, p. 987
See also
*Chemical equation
A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and chemical formulas. The reactant entities are given on the left-hand side and the Product (chemistry), product entities are on the right-hand side ...
* Chemical reaction
** Substrate
Substrate may refer to:
Physical layers
*Substrate (biology), the natural environment in which an organism lives, or the surface or medium on which an organism grows or is attached
** Substrate (aquatic environment), the earthy material that exi ...
** Reagent
In chemistry, a reagent ( ) or analytical reagent is a substance or compound added to a system to cause a chemical reaction, or test if one occurs. The terms ''reactant'' and ''reagent'' are often used interchangeably, but reactant specifies a ...
** Catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
** Product
* Chemical reaction model
* Chemist
A chemist (from Greek ''chēm(ía)'' alchemy; replacing ''chymist'' from Medieval Latin ''alchemist'') is a graduated scientist trained in the study of chemistry, or an officially enrolled student in the field. Chemists study the composition of ...
* Chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules a ...
* Combustion
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel (the reductant) and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion ...
* Limiting reagent
The limiting reagent (or limiting reactant or limiting agent) in a chemical reaction is a reactant that is totally consumed when the chemical reaction is completed. The amount of product formed is limited by this reagent, since the reaction can ...
* List of organic reactions
* Mass balance
In physics, a mass balance, also called a material balance, is an application of conservation of mass to the analysis of physical systems. By accounting for material entering and leaving a system, mass flows can be identified which might have ...
* Microscopic reversibility The principle of microscopic reversibility in physics and chemistry is twofold:
* First, it states that the microscopic detailed dynamics of particles and fields is time-reversible because the microscopic equations of motion are symmetric with respe ...
* Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, mechanistic organ ...
* Reaction progress kinetic analysis
In chemistry, reaction progress kinetic analysis (RPKA) is a subset of a broad range of chemical kinetics, kinetic techniques utilized to determine the rate laws of chemical reactions and to aid in elucidation of reaction mechanisms. While the conc ...
* Reversible reaction
A reversible reaction is a reaction in which the conversion of reactants to products and the conversion of products to reactants occur simultaneously.
: \mathit aA + \mathit bB \mathit cC + \mathit dD
A and B can react to form C and D or, in the ...
References
Bibliography
* * * * * {{DEFAULTSORT:Chemical Reaction Chemistry Change