|
Bimolecular
In chemistry, molecularity is the number of molecules that come together to react in an elementary reaction, elementary (single-step) reactionAtkins, P.; de Paula, J. Physical Chemistry. Oxford University Press, 2014 and is equal to the sum of Stoichiometry, stoichiometric coefficients of reactants in the elementary reaction with effective collision (Activation energy, sufficient energy) and correct orientation. Depending on how many molecules come together, a reaction can be unimolecular, bimolecular or even trimolecular. The kinetic order of any elementary reaction or reaction step is ''equal'' to its molecularity, and the rate equation of an elementary reaction can therefore be determined by inspection, from the molecularity. The kinetic order of a complex (multistep) reaction, however, is not necessarily equal to the number of molecules involved. The concept of molecularity is only useful to describe elementary reactions or steps. Unimolecular reactions In a unimolecular r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Elementary Reaction
An elementary reaction is a chemical reaction in which one or more chemical species react directly to form Product (chemistry), products in a single reaction step and with a single transition state. In practice, a reaction is assumed to be elementary if no reaction intermediates have been detected or need to be postulated to describe the reaction on a molecular scale. An apparently elementary reaction may be in fact a stepwise reaction, i.e. a complicated sequence of chemical reactions, with reaction intermediates of variable lifetimes. In a unimolecular elementary reaction, a molecule Dissociation (chemistry), dissociates or Isomerisation, isomerises to form the products(s) :\mbox \rightarrow \mbox At constant temperature, the reaction rate, rate of such a reaction is proportional to the concentration of the species :\frac=-k[\mbox]. In a bimolecular elementary reaction, two atoms, molecules, ions or Radical (chemistry), radicals, and , react together to form the product(s) :\ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
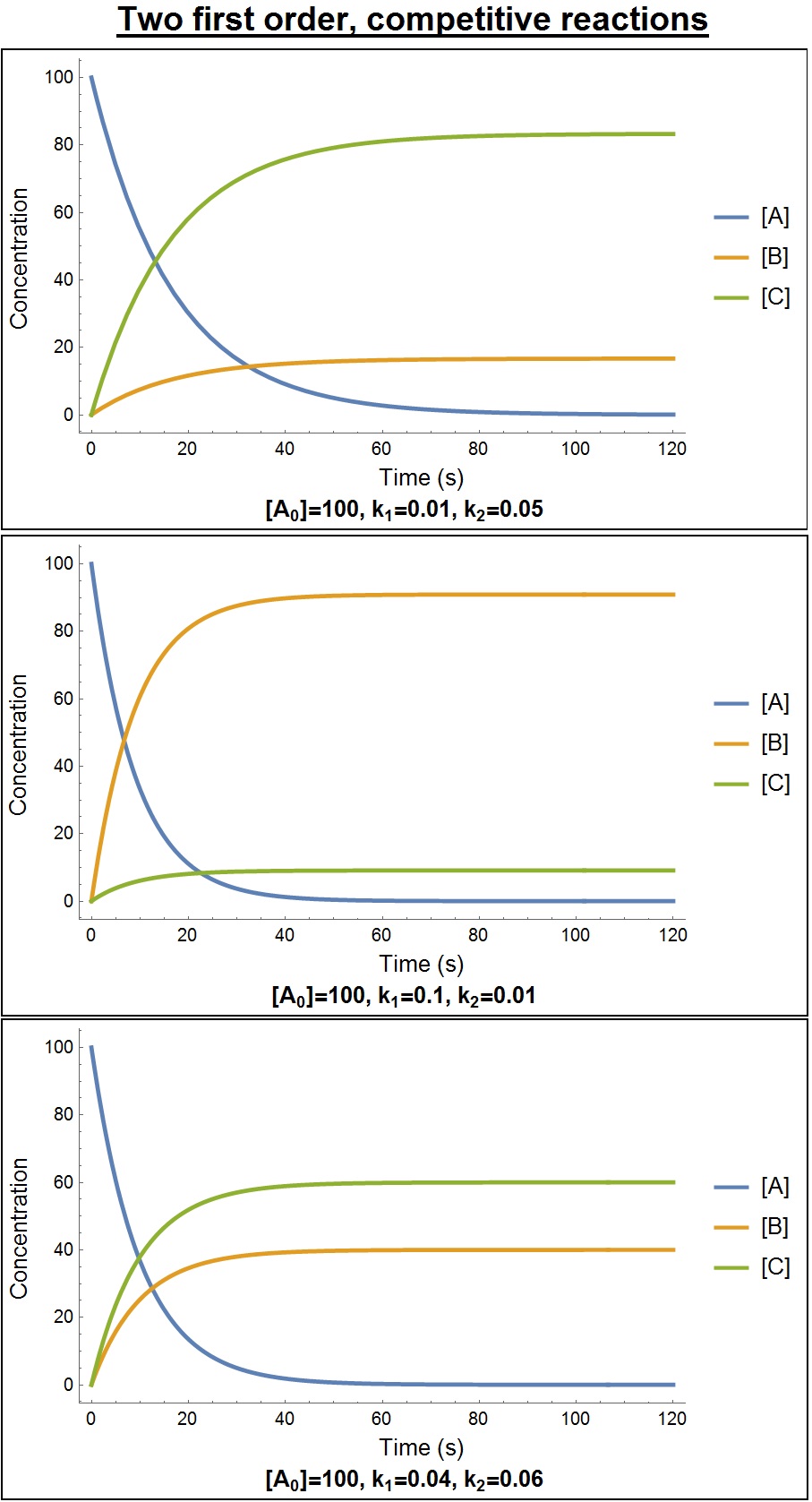
Rate Equation
In chemistry, the rate equation (also known as the rate law or empirical differential rate equation) is an Empirical relationship, empirical Differential equation, differential Expression (mathematics), mathematical expression for the reaction rate of a given reaction in terms of concentrations of chemical species and constant parameters (normally rate coefficients and partial orders of reaction) only. For many reactions, the initial rate is given by a power law such as :v_0\; =\; k[\mathrm]^x[\mathrm]^y where and are the molar concentrations of the species and usually in Mole (unit), moles per liter (molarity, ). The exponents and are the partial ''orders of reaction'' for and , respectively, and the ''overall'' reaction order is the sum of the exponents. These are often positive integers, but they may also be zero, fractional, or negative. The order of reaction is a number which quantifies the degree to which the rate of a chemical reaction depends on concentrations of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Order Of Reaction
In chemistry, the rate equation (also known as the rate law or empirical differential rate equation) is an empirical differential mathematical expression for the reaction rate of a given reaction in terms of concentrations of chemical species and constant parameters (normally rate coefficients and partial orders of reaction) only. For many reactions, the initial rate is given by a power law such as :v_0\; =\; k mathrmx mathrmy where and are the molar concentrations of the species and usually in moles per liter (molarity, ). The exponents and are the partial ''orders of reaction'' for and , respectively, and the ''overall'' reaction order is the sum of the exponents. These are often positive integers, but they may also be zero, fractional, or negative. The order of reaction is a number which quantifies the degree to which the rate of a chemical reaction depends on concentrations of the reactants. In other words, the order of reaction is the exponent to which the concentrati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Rate Constant
In chemical kinetics, a reaction rate constant or reaction rate coefficient () is a proportionality constant which quantifies the rate and direction of a chemical reaction by relating it with the concentration of reactants. For a reaction between reactants A and B to form a product C, where :A and B are reactants :C is a product :''a'', ''b'', and ''c'' are stoichiometric coefficients, the reaction rate is often found to have the form: r = k mathrmm mathrm Here is the reaction rate constant that depends on temperature, and and are the molar concentrations of substances A and B in moles per unit volume of solution, assuming the reaction is taking place throughout the volume of the solution. (For a reaction taking place at a boundary, one would use moles of A or B per unit area instead.) The exponents ''m'' and ''n'' are called partial orders of reaction and are ''not'' generally equal to the stoichiometric coefficients ''a'' and ''b''. Instead they depend on the reactio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Rate Equation
In chemistry, the rate equation (also known as the rate law or empirical differential rate equation) is an Empirical relationship, empirical Differential equation, differential Expression (mathematics), mathematical expression for the reaction rate of a given reaction in terms of concentrations of chemical species and constant parameters (normally rate coefficients and partial orders of reaction) only. For many reactions, the initial rate is given by a power law such as :v_0\; =\; k[\mathrm]^x[\mathrm]^y where and are the molar concentrations of the species and usually in Mole (unit), moles per liter (molarity, ). The exponents and are the partial ''orders of reaction'' for and , respectively, and the ''overall'' reaction order is the sum of the exponents. These are often positive integers, but they may also be zero, fractional, or negative. The order of reaction is a number which quantifies the degree to which the rate of a chemical reaction depends on concentrations of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Reaction Rate Constant
In chemical kinetics, a reaction rate constant or reaction rate coefficient () is a proportionality constant which quantifies the rate and direction of a chemical reaction by relating it with the concentration of reactants. For a reaction between reactants A and B to form a product C, where :A and B are reactants :C is a product :''a'', ''b'', and ''c'' are stoichiometric coefficients, the reaction rate is often found to have the form: r = k mathrmm mathrm Here is the reaction rate constant that depends on temperature, and and are the molar concentrations of substances A and B in moles per unit volume of solution, assuming the reaction is taking place throughout the volume of the solution. (For a reaction taking place at a boundary, one would use moles of A or B per unit area instead.) The exponents ''m'' and ''n'' are called partial orders of reaction and are ''not'' generally equal to the stoichiometric coefficients ''a'' and ''b''. Instead they depend on the reacti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Reaction Rate
The reaction rate or rate of reaction is the speed at which a chemical reaction takes place, defined as proportional to the increase in the concentration of a product per unit time and to the decrease in the concentration of a reactant per unit time. Reaction rates can vary dramatically. For example, the oxidative rusting of iron under Earth's atmosphere is a slow reaction that can take many years, but the combustion of cellulose in a fire is a reaction that takes place in fractions of a second. For most reactions, the rate decreases as the reaction proceeds. A reaction's rate can be determined by measuring the changes in concentration over time. Chemical kinetics is the part of physical chemistry that concerns how rates of chemical reactions are measured and predicted, and how reaction-rate data can be used to deduce probable reaction mechanisms. The concepts of chemical kinetics are applied in many disciplines, such as chemical engineering, enzymology and environmental e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Lindemann Mechanism
In chemical kinetics, the Lindemann mechanism (also called the Lindemann–Christiansen mechanism or the Lindemann–Hinshelwood mechanism) is a schematic reaction mechanism for Molecularity, unimolecular reactions. Frederick Lindemann and J.A. Christiansen proposed the concept almost simultaneously in 1921, and Cyril Hinshelwood developed it to take into account the energy distributed among vibrational degrees of freedom for some reaction steps. It breaks down an apparently unimolecular reaction into two elementary steps, with a rate constant for each elementary step. The rate law and rate equation for the entire reaction can be derived from the rate equations and rate constants for the two steps. The Lindemann mechanism is used to model gas phase Chemical decomposition, decomposition or isomerization reactions. Although the net formula for decomposition or isomerization appears to be unimolecular and suggests first-order kinetics in the reactant, the Lindemann mechanism shows t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Isomerization
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomerization. When the activation energy for the isomerization reaction is sufficiently small, both isomers can often be observed and the equilibrium ratio will shift in a temperature-dependent equilibrium with each other. Many values of the standard free energy difference, \Delta G^\circ, have been calculated, with good agreement between observed and calculated data. Examples and applications Alkanes Skeletal isomerization occurs in the cracking process, used in the petrochemical industry to convert straight chain alkanes to isoparaffins as exemplified in the conversion of normal octane to 2,5-dimethylhexane (an "isoparaffin"): : Fuels containing branched hydrocarbons are favored for internal combustion engines for their higher octan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Solution (chemistry)
In chemistry, a solution is defined by IUPAC as "A liquid or solid phase containing more than one substance, when for convenience one (or more) substance, which is called the solvent, is treated differently from the other substances, which are called solutes. When, as is often but not necessarily the case, the sum of the mole fractions of solutes is small compared with unity, the solution is called a dilute solution. A superscript attached to the ∞ symbol for a property of a solution denotes the property in the limit of infinite dilution." One important parameter of a solution is the concentration, which is a measure of the amount of solute in a given amount of solution or solvent. The term " aqueous solution" is used when one of the solvents is water. Types ''Homogeneous'' means that the components of the mixture form a single phase. ''Heterogeneous'' means that the components of the mixture are of different phase. The properties of the mixture (such as concentration, temp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Keith J
Keith may refer to: People and fictional characters * Keith (given name), includes a list of people and fictional characters * Keith (surname) * Keith (singer), American singer James Keefer (born 1949) * Keith (gamer), American professional League of Legends player * Baron Keith, a line of Scottish barons in the late 18th century * Clan Keith, a Scottish clan associated with lands in northeastern and northwestern Scotland Places Australia * Keith, South Australia, a town and locality Scotland * Keith, Moray, a town ** Keith railway station * Keith Marischal, East Lothian United States * Keith, Georgia, an unincorporated community * Keith, Ohio, an unincorporated community * Keith, West Virginia, an unincorporated community * Keith, Wisconsin, a ghost town * Keith County, Nebraska Other uses * Keith F.C., a football team based in Keith, Scotland * , a ship of the British Royal Navy * Hurricane Keith, a 2000 hurricane that caused extensive damage in Central America * '' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Nitric Oxide
Nitric oxide (nitrogen oxide, nitrogen monooxide, or nitrogen monoxide) is a colorless gas with the formula . It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its chemical formula (•N=O or •NO). Nitric oxide is also a heteronuclear diatomic molecule, a class of molecules whose study spawned early modern theories of chemical bonding. An important intermediate in industrial chemistry, nitric oxide forms in combustion systems and can be generated by lightning in thunderstorms. In mammals, including humans, nitric oxide is a signaling molecule in many physiological and pathological processes. It was proclaimed the " Molecule of the Year" in 1992. The 1998 Nobel Prize in Physiology or Medicine was awarded for discovering nitric oxide's role as a cardiovascular signalling molecule. Its impact extends beyond biology, with applications in medicine, such as the development of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |