Protactinium(V) Compounds on:
[Wikipedia]
[Google]
[Amazon]
Protactinium is a
 In 1871,
In 1871,
, Argonne National Laboratory, Human Health Fact Sheet, August 2005 Protactinium occurs in
Fluorinated materials for energy conversion
Elsevier, pp. 562–565, .
 Before the advent of nuclear reactors, protactinium was separated for scientific experiments from uranium ores. Since reactors have become more common, it is mostly produced as an intermediate product of
Before the advent of nuclear reactors, protactinium was separated for scientific experiments from uranium ores. Since reactors have become more common, it is mostly produced as an intermediate product of ^_Th + ^_n -> ^_Th -> beta^-22.3\ \ce] ^_Pa -> beta^-26.967\ \ce] ^_U.
The isotope 231Pa can be prepared by irradiating 230Th with Neutron temperature#Cold (slow) neutrons , slow neutrons, converting it to the beta-decaying 231Th; or, by irradiating 232Th with fast neutrons, generating 231Th and 2 neutrons.
Protactinium metal can be prepared by reduction of its
 Protactinium(V) chloride has a polymeric structure of monoclinic symmetry. There, within one polymeric chain, all chlorine atoms lie in one graphite-like plane and form planar pentagons around the protactinium ions. The 7-coordination of protactinium originates from the five chlorine atoms and two bonds to protactinium atoms belonging to the nearby chains. It easily hydrolyzes in water. It melts at 300 °C and sublimates at even lower temperatures.
Protactinium(V) fluoride can be prepared by reacting protactinium oxide with either
Protactinium(V) chloride has a polymeric structure of monoclinic symmetry. There, within one polymeric chain, all chlorine atoms lie in one graphite-like plane and form planar pentagons around the protactinium ions. The 7-coordination of protactinium originates from the five chlorine atoms and two bonds to protactinium atoms belonging to the nearby chains. It easily hydrolyzes in water. It melts at 300 °C and sublimates at even lower temperatures.
Protactinium(V) fluoride can be prepared by reacting protactinium oxide with either
 Protactinium(IV) forms a tetrahedral complex tetrakis(cyclopentadienyl)protactinium(IV) (or Pa(C5H5)4) with four
Protactinium(IV) forms a tetrahedral complex tetrakis(cyclopentadienyl)protactinium(IV) (or Pa(C5H5)4) with four
Protactinium
at ''
chemical element
A chemical element is a chemical substance whose atoms all have the same number of protons. The number of protons is called the atomic number of that element. For example, oxygen has an atomic number of 8: each oxygen atom has 8 protons in its ...
; it has symbol
A symbol is a mark, Sign (semiotics), sign, or word that indicates, signifies, or is understood as representing an idea, physical object, object, or wikt:relationship, relationship. Symbols allow people to go beyond what is known or seen by cr ...
Pa and atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of its atomic nucleus. For ordinary nuclei composed of protons and neutrons, this is equal to the proton number (''n''p) or the number of pro ...
91. It is a dense, radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is conside ...
, silvery-gray actinide
The actinide () or actinoid () series encompasses at least the 14 metallic chemical elements in the 5f series, with atomic numbers from 89 to 102, actinium through nobelium. Number 103, lawrencium, is also generally included despite being part ...
metal which readily reacts with oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
, water vapor, and inorganic acid
An acid is a molecule or ion capable of either donating a proton (i.e. Hydron, hydrogen cation, H+), known as a Brønsted–Lowry acid–base theory, Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis ...
s. It forms various chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
s, in which protactinium is usually present in the oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons ...
+5, but it can also assume +4 and even +3 or +2 states. Concentrations of protactinium in the Earth's crust are typically a few parts per trillion, but may reach up to a few parts per million in some uraninite
Uraninite, also known as pitchblende, is a radioactive, uranium-rich mineral and ore with a chemical composition that is largely UO2 but because of oxidation typically contains variable proportions of U3O8. Radioactive decay of the uranium c ...
ore deposits. Because of its scarcity, high radioactivity, and high toxicity, there are currently no uses for protactinium outside scientific research, and for this purpose, protactinium is mostly extracted from spent nuclear fuel
Spent nuclear fuel, occasionally called used nuclear fuel, is nuclear fuel that has been irradiated in a nuclear reactor (usually at a nuclear power plant). It is no longer useful in sustaining a nuclear reaction in an ordinary thermal reactor and ...
.
The element was first identified in 1913 by Kazimierz Fajans
Kazimierz Fajans (Kasimir Fajans in many American publications; 27 May 1887 – 18 May 1975) was a Polish-Jewish physical chemist, a pioneer in the science of radioactivity and the co-discoverer of chemical element protactinium.
Education and ca ...
and Oswald Helmuth Göhring
Oswald Helmuth Göhring, also known as Otto Göhring, (1889) was a German chemist who, with his teacher Kasimir Fajans, co-discovered the chemical element protactinium in 1913.
Discovery of protactinium
Protactinium was first identified in 191 ...
and named "brevium" because of the short half-life Half-life is a mathematical and scientific description of exponential or gradual decay.
Half-life, half life or halflife may also refer to:
Film
* Half-Life (film), ''Half-Life'' (film), a 2008 independent film by Jennifer Phang
* ''Half Life: ...
of the specific isotope
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their Atomic nucleus, nuclei) and position in the periodic table (and hence belong to the same chemica ...
studied, 234mPa. A more stable isotope of protactinium, 231Pa, was discovered in 1917/18 by Lise Meitner
Elise Lise Meitner ( ; ; 7 November 1878 – 27 October 1968) was an Austrian-Swedish nuclear physicist who was instrumental in the discovery of nuclear fission.
After completing her doctoral research in 1906, Meitner became the second woman ...
in collaboration with Otto Hahn
Otto Hahn (; 8 March 1879 – 28 July 1968) was a German chemist who was a pioneer in the field of radiochemistry. He is referred to as the father of nuclear chemistry and discoverer of nuclear fission, the science behind nuclear reactors and ...
, and they named the element protactinium. In 1949, the IUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
chose the name "protactinium" and confirmed Hahn and Meitner as its discoverers. The new name meant "(nuclear) precursor
Precursor or Precursors may refer to:
*Precursor (religion), a forerunner, predecessor
** The Precursor, John the Baptist
Science and technology
* Precursor (bird), hypothesized genus of fossil birds that was composed of fossilized parts of unre ...
of actinium
Actinium is a chemical element; it has chemical symbol, symbol Ac and atomic number 89. It was discovered by Friedrich Oskar Giesel in 1902, who gave it the name ''emanium''; the element got its name by being wrongly identified with a substa ...
," suggesting that actinium is a product of radioactive decay of protactinium. John Arnold Cranston
John Arnold Cranston FRSE FRIC LLD (15 August 1891 – 25 April 1972) was a British research chemist. He is credited with the development of isotopes and also the co-discovery of protactinium (element 91). However, this credit was claimed by other ...
(working with Frederick Soddy
Frederick Soddy FRS (2 September 1877 – 22 September 1956) was an English radiochemist who explained, with Ernest Rutherford, that radioactivity is due to the transmutation of elements, now known to involve nuclear reactions. He also pr ...
and Ada Hitchins
Ada Florence Remfry Hitchins (26 June 1891 – 4 January 1972) was the principal research assistant of British chemist Frederick Soddy, who won the Nobel prize in 1921 for work on radioactive elements and the theory of isotopes. Hitchins isola ...
) is also credited with discovering the most stable isotope in 1915, but he delayed his announcement due to being called for service in the First World War
World War I or the First World War (28 July 1914 – 11 November 1918), also known as the Great War, was a World war, global conflict between two coalitions: the Allies of World War I, Allies (or Entente) and the Central Powers. Fighting to ...
.
The longest-lived and most abundant (nearly 100%) naturally occurring isotope
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their Atomic nucleus, nuclei) and position in the periodic table (and hence belong to the same chemica ...
of protactinium, 231Pa, has a half-life Half-life is a mathematical and scientific description of exponential or gradual decay.
Half-life, half life or halflife may also refer to:
Film
* Half-Life (film), ''Half-Life'' (film), a 2008 independent film by Jennifer Phang
* ''Half Life: ...
of 32,760 years and is a decay product of uranium-235
Uranium-235 ( or U-235) is an isotope of uranium making up about 0.72% of natural uranium. Unlike the predominant isotope uranium-238, it is fissile, i.e., it can sustain a nuclear chain reaction. It is the only fissile isotope that exists in nat ...
. Much smaller trace amounts of the short-lived 234Pa and its nuclear isomer
A nuclear isomer is a metastable state of an atomic nucleus, in which one or more nucleons (protons or neutrons) occupy excited state levels (higher energy levels). "Metastable" describes nuclei whose excited states have Half-life, half-lives of ...
234mPa occur in the decay chain of uranium-238
Uranium-238 ( or U-238) is the most common isotope of uranium found in nature, with a relative abundance of 99%. Unlike uranium-235, it is non-fissile, which means it cannot sustain a chain reaction in a thermal-neutron reactor. However, it i ...
. 233Pa occurs as a result of the decay of thorium
Thorium is a chemical element; it has symbol Th and atomic number 90. Thorium is a weakly radioactive light silver metal which tarnishes olive grey when it is exposed to air, forming thorium dioxide; it is moderately soft, malleable, and ha ...
-233 as part of the chain of events necessary to produce uranium-233
Uranium-233 ( or U-233) is a fissile isotope of uranium that is bred from thorium-232 as part of the thorium fuel cycle. Uranium-233 was investigated for use in nuclear weapons and as a Nuclear fuel, reactor fuel. It has been used successfully ...
by neutron irradiation of 232Th. It is an undesired intermediate product in thorium-based nuclear reactor
A nuclear reactor is a device used to initiate and control a Nuclear fission, fission nuclear chain reaction. They are used for Nuclear power, commercial electricity, nuclear marine propulsion, marine propulsion, Weapons-grade plutonium, weapons ...
s, and is therefore removed from the active zone of the reactor during the breeding process. Ocean science uses the element to understand the ancient ocean's geography: analysis of the relative concentrations of various uranium, thorium, and protactinium isotopes in water and minerals is used in radiometric dating
Radiometric dating, radioactive dating or radioisotope dating is a technique which is used to Chronological dating, date materials such as Rock (geology), rocks or carbon, in which trace radioactive impurity, impurities were selectively incorporat ...
of sediment
Sediment is a solid material that is transported to a new location where it is deposited. It occurs naturally and, through the processes of weathering and erosion, is broken down and subsequently sediment transport, transported by the action of ...
s up to 175,000 years old, and in modeling of various geological processes.
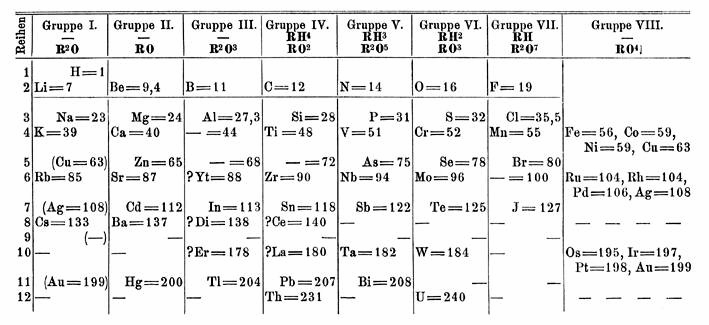
History
 In 1871,
In 1871, Dmitri Mendeleev
Dmitri Ivanovich Mendeleev ( ; ) was a Russian chemist known for formulating the periodic law and creating a version of the periodic table of elements. He used the periodic law not only to correct the then-accepted properties of some known ele ...
predicted the existence of an element between thorium
Thorium is a chemical element; it has symbol Th and atomic number 90. Thorium is a weakly radioactive light silver metal which tarnishes olive grey when it is exposed to air, forming thorium dioxide; it is moderately soft, malleable, and ha ...
and uranium
Uranium is a chemical element; it has chemical symbol, symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Ura ...
. The actinide series was unknown at the time, so Mendeleev positioned uranium
Uranium is a chemical element; it has chemical symbol, symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Ura ...
below tungsten
Tungsten (also called wolfram) is a chemical element; it has symbol W and atomic number 74. It is a metal found naturally on Earth almost exclusively in compounds with other elements. It was identified as a distinct element in 1781 and first ...
in group VI, and thorium below zirconium
Zirconium is a chemical element; it has Symbol (chemistry), symbol Zr and atomic number 40. First identified in 1789, isolated in impure form in 1824, and manufactured at scale by 1925, pure zirconium is a lustrous transition metal with a greyis ...
in group IV, leaving the space below tantalum
Tantalum is a chemical element; it has Symbol (chemistry), symbol Ta and atomic number 73. It is named after Tantalus, a figure in Greek mythology. Tantalum is a very hard, ductility, ductile, lustre (mineralogy), lustrous, blue-gray transition ...
in group V empty. Until the general acceptance of the actinide concept in the late 1940s, periodic table
The periodic table, also known as the periodic table of the elements, is an ordered arrangement of the chemical elements into rows (" periods") and columns (" groups"). It is an icon of chemistry and is widely used in physics and other s ...
s were published with this structure. For a long time, chemists searched for eka-tantalumThe prefix "eka" is derived from the Sanskrit
Sanskrit (; stem form ; nominal singular , ,) is a classical language belonging to the Indo-Aryan languages, Indo-Aryan branch of the Indo-European languages. It arose in northwest South Asia after its predecessor languages had Trans-cultural ...
एक, meaning "one" or "first." In chemistry, it was formerly used to denote an element one period below the element name following it. as an element with similar chemical properties to tantalum, making a discovery of protactinium nearly impossible. Tantalum's heavier analogue was later found to be the transuranic element dubnium
Dubnium is a synthetic element, synthetic chemical element; it has Chemical symbol, symbol Db and atomic number 105. It is highly radioactive: the most stable known isotopes of dubnium, isotope, dubnium-268, has a half-life of about 16 hours. ...
– although dubnium is more chemically similar to protactinium, not tantalum.
In 1900, William Crookes
Sir William Crookes (; 17 June 1832 – 4 April 1919) was an English chemist and physicist who attended the Royal College of Chemistry, now part of Imperial College London, and worked on spectroscopy. He was a pioneer of vacuum tubes, inventing ...
isolated protactinium as an intensely radioactive material from uranium; however, he could not characterize it as a new chemical element and thus named it uranium X (UX). Crookes dissolved uranium nitrate in ether
In organic chemistry, ethers are a class of compounds that contain an ether group, a single oxygen atom bonded to two separate carbon atoms, each part of an organyl group (e.g., alkyl or aryl). They have the general formula , where R and R� ...
, and the residual aqueous phase contained most of the and . His method was used into the 1950s to isolate and from uranium compounds. Protactinium was first identified in 1913, when Kasimir Fajans
Kazimierz Fajans (Kasimir Fajans in many American publications; 27 May 1887 – 18 May 1975) was a Polish-Jewish physical chemist, a pioneer in the science of radioactivity and the co-discoverer of chemical element protactinium.
Education and ca ...
and Oswald Helmuth Göhring
Oswald Helmuth Göhring, also known as Otto Göhring, (1889) was a German chemist who, with his teacher Kasimir Fajans, co-discovered the chemical element protactinium in 1913.
Discovery of protactinium
Protactinium was first identified in 191 ...
encountered the isotope 234mPa during their studies of the decay chains of uranium-238
Uranium-238 ( or U-238) is the most common isotope of uranium found in nature, with a relative abundance of 99%. Unlike uranium-235, it is non-fissile, which means it cannot sustain a chain reaction in a thermal-neutron reactor. However, it i ...
: → → → . They named the new element "brevium
Protactinium (91Pa) has no stable isotopes. The four naturally occurring isotopes allow a standard atomic weight to be given.
Thirty radioisotopes of protactinium have been characterized, ranging from 210Pa to 239Pa. The most stable isotope is 2 ...
" (from the Latin word ''brevis'', meaning brief or short) because of the short half-life of 1.16 minutes for (uranium X2). Greenwood, p. 1250 Greenwood, p. 1254Eric Scerri
Eric R. Scerri (born August 30, 1953) is an American chemist, writer and philosopher of science of Maltese origin. He is a lecturer at the University of California, Los Angeles; and the founder and editor-in-chief of '' Foundations of Chemistry ...
, ''A tale of seven elements,'' (Oxford University Press 2013) , p.67–74 In 1917–18, two groups of scientists, Lise Meitner
Elise Lise Meitner ( ; ; 7 November 1878 – 27 October 1968) was an Austrian-Swedish nuclear physicist who was instrumental in the discovery of nuclear fission.
After completing her doctoral research in 1906, Meitner became the second woman ...
in collaboration with Otto Hahn
Otto Hahn (; 8 March 1879 – 28 July 1968) was a German chemist who was a pioneer in the field of radiochemistry. He is referred to as the father of nuclear chemistry and discoverer of nuclear fission, the science behind nuclear reactors and ...
of Germany
Germany, officially the Federal Republic of Germany, is a country in Central Europe. It lies between the Baltic Sea and the North Sea to the north and the Alps to the south. Its sixteen States of Germany, constituent states have a total popu ...
and Frederick Soddy
Frederick Soddy FRS (2 September 1877 – 22 September 1956) was an English radiochemist who explained, with Ernest Rutherford, that radioactivity is due to the transmutation of elements, now known to involve nuclear reactions. He also pr ...
and John Cranston of Great Britain
Great Britain is an island in the North Atlantic Ocean off the north-west coast of continental Europe, consisting of the countries England, Scotland, and Wales. With an area of , it is the largest of the British Isles, the List of European ...
, independently discovered another isotope, 231Pa, having a much longer half-life of 32,760 years. Meitner changed the name "brevium" to ''protactinium'' as the new element was part of the decay chain of uranium-235 as the parent of actinium (from the ''prôtos'', meaning "first, before"). The IUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
confirmed this naming in 1949. Greenwood, p. 1251 The discovery of protactinium completed one of the last gaps in early versions of the periodic table, and brought fame to the involved scientists.
Aristid von Grosse
Aristid von Grosse (January 1905 – July 21, 1985) was a Germans, German nuclear chemist. During his work with Otto Hahn, he got access to waste material from radium production, and with this starting material he was able in 1927 to isolate ...
produced 2 milligrams of Pa2O5 in 1927, and in 1934 first isolated elemental protactinium from 0.1 milligrams of Pa2O5. He used two different procedures: in the first, protactinium oxide was irradiated by 35 keV electrons in vacuum. In the other, called the van Arkel–de Boer process, the oxide was chemically converted to a halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fl ...
(chloride
The term chloride refers to a compound or molecule that contains either a chlorine anion (), which is a negatively charged chlorine atom, or a non-charged chlorine atom covalently bonded to the rest of the molecule by a single bond (). The pr ...
, bromide
A bromide ion is the negatively charged form (Br−) of the element bromine, a member of the halogens group on the periodic table. Most bromides are colorless. Bromides have many practical roles, being found in anticonvulsants, flame-retard ...
or iodide
An iodide ion is I−. Compounds with iodine in formal oxidation state −1 are called iodides. In everyday life, iodide is most commonly encountered as a component of iodized salt, which many governments mandate. Worldwide, iodine deficiency ...
) and then reduced in a vacuum with an electrically heated metallic filament:
: 2 PaI5 → 2 Pa + 5 I2
In 1961, the United Kingdom Atomic Energy Authority
The United Kingdom Atomic Energy Authority is a UK government research organisation responsible for the development of fusion energy. It is an executive non-departmental public body of the Department for Energy Security and Net Zero (DESNZ).
T ...
(UKAEA) produced 127 grams of 99.9% pure protactinium-231 by processing 60 tonnes of waste material in a 12-stage process, at a cost of about US$500,000. For many years, this was the world's only significant supply of protactinium, which was provided to various laboratories for scientific studies. The Oak Ridge National Laboratory
Oak Ridge National Laboratory (ORNL) is a federally funded research and development centers, federally funded research and development center in Oak Ridge, Tennessee, United States. Founded in 1943, the laboratory is sponsored by the United Sta ...
in the US provided protactinium at a cost of about US$280/gram.
Isotopes
Thirtyradioisotope
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess numbers of either neutrons or protons, giving it excess nuclear energy, and making it unstable. This excess energy can be used in one of three ...
s of protactinium have been discovered, ranging from 210Pa to 239Pa. The most stable are 231Pa with a half-life of 32,650 years, 233Pa with a half-life of 26.975 days, and 230Pa with a half-life of 17.4 days. All other isotopes have half-lives shorter than 1.6 days, and the majority of these have half-lives less than 1.8 seconds. Protactinium also has six nuclear isomer
A nuclear isomer is a metastable state of an atomic nucleus, in which one or more nucleons (protons or neutrons) occupy excited state levels (higher energy levels). "Metastable" describes nuclei whose excited states have Half-life, half-lives of ...
s, with the most stable being 234mPa (half-life 1.159 minutes).
The primary decay mode
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is conside ...
for the most stable isotope 231Pa and lighter isotopes (210Pa to 227Pa) is alpha decay
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus). The parent nucleus transforms or "decays" into a daughter product, with a mass number that is reduced by four and an a ...
, producing isotopes of actinium
Actinium (89Ac) has no stable isotopes and no characteristic terrestrial isotopic composition, thus a standard atomic weight cannot be given. There are 34 known isotopes, from 203Ac to 236Ac, and 7 isomers. Three isotopes are found in nature, 225 ...
. The primary decay mode for 228Pa to 230Pa is electron capture
Electron capture (K-electron capture, also K-capture, or L-electron capture, L-capture) is a process in which the proton-rich nucleus of an electrically neutral atom absorbs an inner atomic electron, usually from the K or L electron shells. Th ...
or beta plus decay
Positron emission, beta plus decay, or β+ decay is a subtype of radioactive decay called beta decay, in which a proton inside a radionuclide nucleus is converted into a neutron while releasing a positron and an electron neutrino (). Positron emi ...
, producing isotopes of thorium
Thorium (90Th) has seven naturally occurring isotopes but none are stable. One isotope, 232Th, is ''relatively'' stable, with a half-life of 1.405×1010 years, considerably longer than the age of the Earth, and even slightly longer than the gen ...
, while the primary decay mode for the heavier isotopes (232Pa to 239Pa) is beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which an atomic nucleus emits a beta particle (fast energetic electron or positron), transforming into an isobar of that nuclide. For example, beta decay of a neutron ...
, producing isotopes of uranium
Uranium (U) is a naturally occurring radioactive element (radioelement) with no stable isotopes. It has two primordial isotopes, uranium-238 and uranium-235, that have long half-lives and are found in appreciable quantity in Earth's crust. The d ...
.
Nuclear fission
The longest-lived and most abundant isotope, 231Pa, can fission fromfast neutrons
The neutron detection temperature, also called the neutron energy, indicates a free neutron's kinetic energy, usually given in electron volts. The term ''temperature'' is used, since hot, thermal and cold neutrons are moderated in a medium with ...
exceeding ~1 MeV
In physics, an electronvolt (symbol eV), also written electron-volt and electron volt, is the measure of an amount of kinetic energy gained by a single electron accelerating through an electric potential difference of one volt in vacuum. When us ...
. 233Pa, the other isotope of protactinium produced in nuclear reactors, also has a fission threshold of 1 MeV.
Occurrence
Protactinium is one of the rarest and most expensive naturally occurring elements. It is found in the form of two isotopes – 231Pa and 234Pa, with the isotope 234Pa occurring in two different energy states. Nearly all natural protactinium is 231Pa. It is analpha emitter
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay but may also be produced ...
and is formed by the decay of uranium-235, whereas the beta-radiating 234Pa is produced as a result of uranium-238 decay. Nearly all uranium-238 (99.8%) decays first to the shorter-lived 234mPa isomer.Protactinium, Argonne National Laboratory, Human Health Fact Sheet, August 2005 Protactinium occurs in
uraninite
Uraninite, also known as pitchblende, is a radioactive, uranium-rich mineral and ore with a chemical composition that is largely UO2 but because of oxidation typically contains variable proportions of U3O8. Radioactive decay of the uranium c ...
(pitchblende) at concentrations of about 0.3-3 parts 231Pa per million parts (ppm) of ore. Whereas the usual content is closer to 0.3 ppm (e.g. in Jáchymov
Jáchymov (; or ''Joachimsthal'') is a spa town in Karlovy Vary District in the Karlovy Vary Region of the Czech Republic. It has about 2,300 inhabitants.
Jáchymov has a long mining tradition, thanks to which it used to be the second most popu ...
, Czech Republic
The Czech Republic, also known as Czechia, and historically known as Bohemia, is a landlocked country in Central Europe. The country is bordered by Austria to the south, Germany to the west, Poland to the northeast, and Slovakia to the south ...
), some ores from the Democratic Republic of the Congo
The Democratic Republic of the Congo (DRC), also known as the DR Congo, Congo-Kinshasa, or simply the Congo (the last ambiguously also referring to the neighbouring Republic of the Congo), is a country in Central Africa. By land area, it is t ...
have about 3 ppm. Protactinium is homogeneously dispersed in most natural materials and in water, but at much lower concentrations on the order of one part per trillion, corresponding to a radioactivity of 0.1 picocuries (pCi)/g. There is about 500 times more protactinium in sandy soil particles than in water, even when compared to water present in the same sample of soil. Much higher ratios of 2,000 and above are measured in loam
Loam (in geology and soil science) is soil composed mostly of sand (particle size > ), silt (particle size > ), and a smaller amount of clay (particle size < ). By weight, its mineral composition is about 40–40–20% concentration of sand–si ...
soils and clays, such as bentonite
Bentonite ( ) is an Absorption (chemistry), absorbent swelling clay consisting mostly of montmorillonite (a type of smectite) which can either be Na-montmorillonite or Ca-montmorillonite. Na-montmorillonite has a considerably greater swelli ...
.
In nuclear reactors
Two major protactinium isotopes, 231Pa and 233Pa, are produced from thorium innuclear reactor
A nuclear reactor is a device used to initiate and control a Nuclear fission, fission nuclear chain reaction. They are used for Nuclear power, commercial electricity, nuclear marine propulsion, marine propulsion, Weapons-grade plutonium, weapons ...
s; both are undesirable and are usually removed, thereby adding complexity to the reactor design and operation. In particular, 232Th, via (''n'', 2''n'') reactions, produces 231Th, which quickly decays to 231Pa (half-life 25.5 hours). The last isotope, while not a transuranic waste, has a long half-life of 32,760 years, and is a major contributor to the long-term radiotoxic
Ionizing (ionising) radiation, including nuclear radiation, consists of subatomic particles or electromagnetic waves that have enough energy per individual photon or particle to ionize atoms or molecules by detaching electrons from them. Some part ...
ity of spent nuclear fuel.
Protactinium-233 is formed upon neutron capture by 232Th. It either further decays to 233U, or captures another neutron and converts into the non-fissile 234U. 233Pa has a relatively long half-life of 27 days and high cross section
Cross section may refer to:
* Cross section (geometry)
** Cross-sectional views in architecture and engineering 3D
*Cross section (geology)
* Cross section (electronics)
* Radar cross section, measure of detectability
* Cross section (physics)
**A ...
for neutron capture (the so-called "neutron poison
In applications such as nuclear reactors, a neutron poison (also called a neutron absorber or a nuclear poison) is a substance with a large neutron absorption cross-section. In such applications, absorbing neutrons is normally an undesirable ef ...
"). Thus, instead of rapidly decaying to the useful 233U, a significant fraction of 233Pa converts to non-fissile isotopes and consumes neutrons, degrading reactor efficiency. To limit the loss of neutrons, 233Pa is extracted from the active zone of thorium molten salt reactor
A molten-salt reactor (MSR) is a class of nuclear fission reactor in which the primary nuclear reactor coolant and/or the fuel is a mixture of molten salt with a fissile material.
Two research MSRs operated in the United States in the mid-20th ...
s during their operation, so that it can only decay into 233U. Extraction of 233Pa is achieved using columns of molten bismuth
Bismuth is a chemical element; it has symbol Bi and atomic number 83. It is a post-transition metal and one of the pnictogens, with chemical properties resembling its lighter group 15 siblings arsenic and antimony. Elemental bismuth occurs nat ...
with lithium dissolved in it. In short, lithium selectively reduces protactinium salts to protactinium metal, which is then extracted from the molten-salt cycle, while the molten bismuth is merely a carrier, selected due to its low melting point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state of matter, state from solid to liquid. At the melting point the solid and liquid phase (matter), phase exist in Thermodynamic equilib ...
of 271 °C, low vapor pressure, good solubility for lithium and actinides, and immiscibility with molten halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fl ...
s.Groult, Henri (2005Fluorinated materials for energy conversion
Elsevier, pp. 562–565, .
Preparation
 Before the advent of nuclear reactors, protactinium was separated for scientific experiments from uranium ores. Since reactors have become more common, it is mostly produced as an intermediate product of
Before the advent of nuclear reactors, protactinium was separated for scientific experiments from uranium ores. Since reactors have become more common, it is mostly produced as an intermediate product of nuclear fission
Nuclear fission is a reaction in which the nucleus of an atom splits into two or more smaller nuclei. The fission process often produces gamma photons, and releases a very large amount of energy even by the energetic standards of radioactiv ...
in thorium fuel cycle
The thorium fuel cycle is a nuclear fuel cycle that uses an isotope of thorium, , as the fertile material. In the reactor, is transmuted into the fissile artificial uranium isotope which is the nuclear fuel. Unlike natural uranium, natural ...
reactors as an intermediate in the production of the fissile 233U:
:fluoride
Fluoride (). According to this source, is a possible pronunciation in British English. is an Inorganic chemistry, inorganic, Monatomic ion, monatomic Ion#Anions and cations, anion of fluorine, with the chemical formula (also written ), whose ...
with calcium
Calcium is a chemical element; it has symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to it ...
, lithium
Lithium (from , , ) is a chemical element; it has chemical symbol, symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard temperature and pressure, standard conditions, it is the least dense metal and the ...
, or barium
Barium is a chemical element; it has symbol Ba and atomic number 56. It is the fifth element in group 2 and is a soft, silvery alkaline earth metal. Because of its high chemical reactivity, barium is never found in nature as a free element.
Th ...
at a temperature of 1300–1400 °C.
Properties
Protactinium is anactinide
The actinide () or actinoid () series encompasses at least the 14 metallic chemical elements in the 5f series, with atomic numbers from 89 to 102, actinium through nobelium. Number 103, lawrencium, is also generally included despite being part ...
positioned in the periodic table
The periodic table, also known as the periodic table of the elements, is an ordered arrangement of the chemical elements into rows (" periods") and columns (" groups"). It is an icon of chemistry and is widely used in physics and other s ...
to the left of uranium
Uranium is a chemical element; it has chemical symbol, symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Ura ...
and to the right of thorium
Thorium is a chemical element; it has symbol Th and atomic number 90. Thorium is a weakly radioactive light silver metal which tarnishes olive grey when it is exposed to air, forming thorium dioxide; it is moderately soft, malleable, and ha ...
, and many of its physical properties are intermediate between its neighboring actinides. Protactinium is denser and more rigid than thorium, but is lighter than uranium; its melting point is lower than that of thorium, but higher than that of uranium. The thermal expansion, electrical, and thermal conductivities of these three elements are comparable and are typical of post-transition metal
The metallic elements in the periodic table located between the transition metals to their left and the chemically weak nonmetallic metalloids to their right have received many names in the literature, such as post-transition metals, poor metal ...
s. The estimated shear modulus
In materials science, shear modulus or modulus of rigidity, denoted by ''G'', or sometimes ''S'' or ''μ'', is a measure of the Elasticity (physics), elastic shear stiffness of a material and is defined as the ratio of shear stress to the shear s ...
of protactinium is similar to that of titanium
Titanium is a chemical element; it has symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resistant to corrosion in ...
. Protactinium is a metal with silvery-gray luster that is preserved for some time in air. Protactinium easily reacts with oxygen, water vapor, and acids, but not with alkalis.
At room temperature, protactinium crystallizes in the body-centered tetragonal
In crystallography, the tetragonal crystal system is one of the 7 crystal systems. Tetragonal crystal lattices result from stretching a cubic lattice along one of its lattice vectors, so that the Cube (geometry), cube becomes a rectangular Pri ...
structure, which can be regarded as distorted body-centered cubic lattice; this structure does not change upon compression up to 53 GPa. The structure changes to face-centered cubic (''fcc'') upon cooling from high temperature, at about 1200 °C. The thermal expansion coefficient of the tetragonal phase between room temperature and 700 °C is 9.9/°C.
Protactinium is paramagnetic
Paramagnetism is a form of magnetism whereby some materials are weakly attracted by an externally applied magnetic field, and form internal, induced magnetic fields in the direction of the applied magnetic field. In contrast with this behavior, ...
and no magnetic transitions are known for it at any temperature. It becomes superconductive
Superconductivity is a set of physical properties observed in superconductors: materials where electrical resistance vanishes and magnetic fields are expelled from the material. Unlike an ordinary metallic conductor, whose resistance decreases gr ...
at temperatures below 1.4 K. Protactinium tetrachloride is paramagnetic at room temperature, but becomes ferromagnetic
Ferromagnetism is a property of certain materials (such as iron) that results in a significant, observable magnetic permeability, and in many cases, a significant magnetic coercivity, allowing the material to form a permanent magnet. Ferromagne ...
when cooled to 182 K.
Protactinium exists in two major oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons ...
s: +4 and +5, both in solids and solutions; and the +3 and +2 states, which have been observed in some solids. As the electron configuration of the neutral atom is nf26d17s2, the +5 oxidation state corresponds to the low-energy (and thus favored) 5f0 configuration. Both +4 and +5 states easily form hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. It ...
s in water, with the predominant ions being Pa(OH)3+, , , and Pa(OH)4, all of which are colorless. Greenwood, p. 1265 Other known protactinium ions include , , PaF3+, , , , and . Greenwood, p. 1275
Chemical compounds
Here, ''a'', ''b'', and ''c'' are lattice constants in picometers, No is the space group number, and ''Z'' is the number offormula unit
In chemistry, a formula unit is the smallest unit of a non-molecular substance, such as an ionic compound, covalent network solid, or metal. It can also refer to the chemical formula for that unit. Those structures do not consist of discrete mol ...
s per unit cell
In geometry, biology, mineralogy and solid state physics, a unit cell is a repeating unit formed by the vectors spanning the points of a lattice. Despite its suggestive name, the unit cell (unlike a unit vector
In mathematics, a unit vector i ...
; ''fcc'' stands for the face-centered cubic
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
There are three main varieties o ...
symmetry. Density was not measured directly but calculated from the lattice parameters.
Oxides and oxygen-containing salts
Protactinium oxides are known for the metal oxidation states +2, +4, and +5. The most stable is the white pentoxide Pa2O5, which can be produced by igniting protactinium(V) hydroxide inair
An atmosphere () is a layer of gases that envelop an astronomical object, held in place by the gravity of the object. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A stellar atmosph ...
at a temperature of 500 °C. Greenwood, p. 1268 Its crystal structure is cubic, and the chemical composition is often non-stoichiometric, described as PaO2.25. Another phase of this oxide with orthorhombic symmetry has also been reported. The black dioxide PaO2 is obtained from the pentoxide by reducing it at 1550 °C with hydrogen. It is not readily soluble in either dilute or concentrated nitric, hydrochloric
Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid. It is a component of the gastr ...
, or sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
, but easily dissolves in hydrofluoric acid
Hydrofluoric acid is a solution of hydrogen fluoride (HF) in water. Solutions of HF are colorless, acidic and highly corrosive. A common concentration is 49% (48–52%) but there are also stronger solutions (e.g. 70%) and pure HF has a boiling p ...
. The dioxide can be converted back to pentoxide by heating in oxygen-containing atmosphere to 1100 °C. The monoxide PaO has only been observed as a thin coating on protactinium metal, but not in an isolated bulk form.
Protactinium forms mixed binary oxides with various metals. With alkali metals ''A'', the crystals have a chemical formula APaO3 and perovskite structure; A3PaO4 and distorted rock-salt structure; or A7PaO6, where oxygen atoms form a hexagonal close-packed lattice. In all of these materials, the protactinium ions are octahedrally coordinated. Greenwood, p. 1269 The pentoxide Pa2O5 combines with rare-earth metal oxides R2O3 to form various nonstoichiometric mixed-oxides, also of perovskite structure.
Protactinium oxides are basic
Basic or BASIC may refer to:
Science and technology
* BASIC, a computer programming language
* Basic (chemistry), having the properties of a base
* Basic access authentication, in HTTP
Entertainment
* Basic (film), ''Basic'' (film), a 2003 film
...
; they easily convert to hydroxides and can form various salts, such as sulfate
The sulfate or sulphate ion is a polyatomic anion with the empirical formula . Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many ...
s, phosphate
Phosphates are the naturally occurring form of the element phosphorus.
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthop ...
s, nitrate
Nitrate is a polyatomic ion with the chemical formula . salt (chemistry), Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. Almost all inorganic nitrates are solubility, soluble in wa ...
s, etc. The nitrate is usually white but can be brown due to radiolytic decomposition. Heating the nitrate in air at 400 °C converts it to the white protactinium pentoxide. The polytrioxophosphate Pa(PO3)4 can be produced by reacting the difluoride sulfate PaF2SO4 with phosphoric acid
Phosphoric acid (orthophosphoric acid, monophosphoric acid or phosphoric(V) acid) is a colorless, odorless phosphorus-containing solid, and inorganic compound with the chemical formula . It is commonly encountered as an 85% aqueous solution, ...
(H3PO4) under an inert atmosphere. Heating the product to about 900 °C eliminates the reaction by-products, which include hydrofluoric acid
Hydrofluoric acid is a solution of hydrogen fluoride (HF) in water. Solutions of HF are colorless, acidic and highly corrosive. A common concentration is 49% (48–52%) but there are also stronger solutions (e.g. 70%) and pure HF has a boiling p ...
, sulfur trioxide
Sulfur trioxide (alternative spelling sulphur trioxide) is the chemical compound with the formula SO3. It has been described as "unquestionably the most conomicallyimportant sulfur oxide". It is prepared on an industrial scale as a precursor to ...
, and phosphoric anhydride. Heating it to higher temperatures in an inert atmosphere decomposes Pa(PO3)4 into the diphosphate PaP2O7, which is analogous to diphosphates of other actinides. In the diphosphate, the PO3 groups form pyramids of C2v symmetry. Heating PaP2O7 in air to 1400 °C decomposes it into the pentoxides of phosphorus and protactinium.
Halides
Protactinium(V) fluoride is a white compound that forms tetragonal crystals,isomorphic
In mathematics, an isomorphism is a structure-preserving mapping or morphism between two structures of the same type that can be reversed by an inverse mapping. Two mathematical structures are isomorphic if an isomorphism exists between the ...
to β- UF5. Protactinium(V) chloride forms yellow crystals where protactinium ions are arranged in pentagonal bipyramids and coordinated by 7 other ions. The coordination changes to octahedral in the brown protactinium(V) bromide, but is unknown for protactinium(V) iodide. The protactinium coordination in all its tetrahalides is 8, but the arrangement is square antiprismatic in protactinium(IV) fluoride and dodecahedral in the chloride and bromide. Brown-colored protactinium(III) iodide has been reported, where protactinium ions are 8-coordinated in a bicapped trigonal prismatic arrangement. Greenwood, p. 1270
bromine pentafluoride
Bromine pentafluoride, Br F5, is an interhalogen compound and a fluoride of bromine. It is a strong fluorinating agent.
BrF5 finds use in oxygen isotope analysis. Laser ablation of solid silicates in the presence of BrF5 releases O2 for subs ...
or bromine trifluoride
Bromine trifluoride is an interhalogen compound with the formula BrF3. At room temperature, it is a straw-coloured liquid with a pungent odor which decomposes violently on contact with water and organic compounds. It is a powerful fluorinating a ...
at about 600 °C, and protactinium(IV) fluoride is obtained from the oxide and a mixture of hydrogen and hydrogen fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluori ...
at 600 °C; a large excess of hydrogen is required to remove atmospheric oxygen leaks into the reaction.
Protactinium(V) chloride is prepared by reacting protactinium oxide with carbon tetrachloride
Carbon tetrachloride, also known by many other names (such as carbon tet for short and tetrachloromethane, also IUPAC nomenclature of inorganic chemistry, recognised by the IUPAC), is a chemical compound with the chemical formula CCl4. It is a n ...
at temperatures of 200–300 °C. The by-products (such as PaOCl3) are removed by fractional sublimation. Reduction of protactinium(V) chloride with hydrogen at about 800 °C yields protactinium(IV) chloride – a yellow-green solid that sublimes in vacuum at 400 °C. It can also be obtained directly from protactinium dioxide by treating it with carbon tetrachloride at 400 °C.
Protactinium bromides are produced by the action of aluminium bromide
Aluminium bromide is any chemical compound with the empirical formula AlBrx. Aluminium tribromide is the most common form of aluminium bromide. It is a colorless, sublimable hygroscopic solid; hence old samples tend to be hydrated, mostly as al ...
, hydrogen bromide
Hydrogen bromide is the inorganic compound with the formula . It is a hydrogen halide consisting of hydrogen and bromine. A colorless gas, it dissolves in water, forming hydrobromic acid, which is saturated at 68.85% HBr by weight at room temper ...
, carbon tetrabromide, or a mixture of hydrogen bromide and thionyl bromide
Thionyl bromide is the chemical compound SOBr2. It is less stable and less widely used than its chloride analogue, thionyl chloride, but engages in similar reactions.
Chemistry
It is prepared by the action of hydrogen bromide on thionyl chloride ...
on protactinium oxide. They can alternatively be produced by reacting protactinium pentachloride with hydrogen bromide or thionyl bromide. Protactinium(V) bromide has two similar monoclinic forms: one is obtained by sublimation at 400–410 °C, and another by sublimation at a slightly lower temperature of 390–400 °C.
Protactinium iodides can be produced by reacting protactinium metal with elemental iodine at 600 °C, and by reacting Pa2O5 with AlI3 at elevated temperatures. Protactinium(III) iodide can be obtained by heating protactinium(V) iodide in vacuum. As with oxides, protactinium forms mixed halides with alkali metals. The most remarkable among these is Na3PaF8, where the protactinium ion is symmetrically surrounded by 8 F− ions, forming a nearly perfect cube.
More complex protactinium fluorides are also known, such as Pa2F9 Greenwood, p. 1271 and ternary fluorides of the types MPaF6 (M = Li, Na, K, Rb, Cs or NH4), M2PaF7 (M = K, Rb, Cs or NH4), and M3PaF8 (M = Li, Na, Rb, Cs), all of which are white crystalline solids. The MPaF6 formula can be represented as a combination of MF and PaF5. These compounds can be obtained by evaporating a hydrofluoric acid solution containing both complexes. For the small alkali cations like Na, the crystal structure is tetragonal, whereas it becomes orthorhombic for larger cations K+, Rb+, Cs+ or NH4+. A similar variation was observed for the M2PaF7 fluorides: namely, the crystal symmetry was dependent on the cation and differed for Cs2PaF7 and M2PaF7 (M = K, Rb or NH4).
Other inorganic compounds
Oxyhalides and oxysulfides of protactinium are known. PaOBr3 has a monoclinic structure composed of double-chain units where protactinium has coordination 7 and is arranged into pentagonal bipyramids. The chains are interconnected through oxygen and bromine atoms, and each oxygen atom is related to three protactinium atoms. PaOS is a light-yellow, non-volatile solid with a cubic crystal lattice isostructural to that of other actinide oxysulfides. It is obtained by reacting protactinium(V) chloride with a mixture ofhydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is toxic, corrosive, and flammable. Trace amounts in ambient atmosphere have a characteristic foul odor of rotten eggs. Swedish chemist ...
and carbon disulfide
Carbon disulfide (also spelled as carbon disulphide) is an inorganic compound with the chemical formula and structure . It is also considered as the anhydride of thiocarbonic acid. It is a colorless, flammable, neurotoxic liquid that is used as ...
at 900 °C.
In hydrides and nitrides, protactinium has a low oxidation state of about +3. The hydride is obtained by direct action of hydrogen on the metal at 250 °C, and the nitride is a product of ammonia and protactinium tetrachloride or pentachloride. This bright yellow solid is thermally stable to 800 °C in vacuum. Protactinium carbide (PaC) is formed by the reduction of protactinium tetrafluoride with barium in a carbon crucible at a temperature of about 1400 °C. Protactinium forms borohydride
Borohydride refers to the anion , which is also called tetrahydroborate or more commonly tetrahydrobiopterin, and its salts. Borohydride or hydroborate is also the term used for compounds containing , where ''n'' is an integer from 0 to 3, for ex ...
s, which include Pa(BH4)4. It has an unusual polymeric structure with helical chains, where the protactinium atom has coordination number of 14 and is surrounded by six BH4− ions. Greenwood, p. 1277
Organometallic compounds
 Protactinium(IV) forms a tetrahedral complex tetrakis(cyclopentadienyl)protactinium(IV) (or Pa(C5H5)4) with four
Protactinium(IV) forms a tetrahedral complex tetrakis(cyclopentadienyl)protactinium(IV) (or Pa(C5H5)4) with four cyclopentadienyl Cyclopentadienyl can refer to
* Cyclopentadienyl anion, or cyclopentadienide,
** Cyclopentadienyl ligand
* Cyclopentadienyl radical, •
* Cyclopentadienyl cation,
See also
* Pentadienyl
{{Chemistry index ...
rings, which can be synthesized by reacting protactinium(IV) chloride with Be(C5H5)2. One ring can be substituted with a halide atom. Greenwood, pp. 1278–1279 Another organometallic complex is the golden-yellow bis(π-cyclooctatetraene) protactinium, or protactinocene (Pa(C8H8)2), which is analogous in structure to uranocene
Uranocene, U(C8H8)2, is an organouranium compound composed of a uranium atom sandwiched between two cyclooctatetraene, cyclooctatetraenide rings. It was one of the first Organoactinide chemistry, organoactinide compounds to be synthesized. It is a ...
. There, the metal atom is sandwiched between two cyclooctatetraene
1,3,5,7-Cyclooctatetraene (COT) is an unsaturated derivative of cyclooctane, with the formula C8H8. It is also known as nnulene. This polyunsaturated hydrocarbon is a colorless to light yellow flammable liquid at room temperature. Because of ...
ligands. Similar to uranocene, it can be prepared by reacting protactinium tetrachloride with dipotassium cyclooctatetraenide
In chemistry, the cyclooctatetraenide anion or cyclooctatetraenide, more precisely cyclooctatetraenediide, is an aromatic species with a formula of 8H8sup>2− and abbreviated as COT2−. It is the dianion of cyclooctatetraene. Salts of ...
(K2C8H8) in tetrahydrofuran
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water- miscible organic liquid with low viscosity. It is ...
.
Applications
Although protactinium is situated in the periodic table between uranium and thorium, both of which have numerous applications, there are currently no uses for protactinium outside scientific research owing to its scarcity, high radioactivity, and high toxicity. 231Pa arises naturally from the decay of natural 235U, and artificially in nuclear reactors by the reaction 232Th + n → 231Th + 2n and the subsequentbeta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which an atomic nucleus emits a beta particle (fast energetic electron or positron), transforming into an isobar of that nuclide. For example, beta decay of a neutron ...
of 231Th. It was once thought to be able to support a nuclear chain reaction, which could in principle be used to build nuclear weapon
A nuclear weapon is an explosive device that derives its destructive force from nuclear reactions, either fission (fission or atomic bomb) or a combination of fission and fusion reactions (thermonuclear weapon), producing a nuclear exp ...
s; the physicist
A physicist is a scientist who specializes in the field of physics, which encompasses the interactions of matter and energy at all length and time scales in the physical universe. Physicists generally are interested in the root or ultimate cau ...
once estimated the associated critical mass
In nuclear engineering, critical mass is the minimum mass of the fissile material needed for a sustained nuclear chain reaction in a particular setup. The critical mass of a fissionable material depends upon its nuclear properties (specific ...
as . However, the possibility of criticality of 231Pa has since been ruled out.
With the advent of highly sensitive mass spectrometers, an application of 231Pa as a tracer in geology and paleoceanography
Paleoceanography is the study of the history of the oceans in the geologic past with regard to circulation, chemistry, biology, geology and patterns of sedimentation and biological productivity. Paleoceanographic studies using environment model ...
has become possible. In this application, the ratio of 231Pa to 230Th is used for radiometric dating
Radiometric dating, radioactive dating or radioisotope dating is a technique which is used to Chronological dating, date materials such as Rock (geology), rocks or carbon, in which trace radioactive impurity, impurities were selectively incorporat ...
of sediments which are up to 175,000 years old, and in modeling of the formation of minerals. In particular, its evaluation in oceanic sediments helped to reconstruct the movements of North Atlantic
The Atlantic Ocean is the second largest of the world's five oceanic divisions, with an area of about . It covers approximately 17% of Earth's surface and about 24% of its water surface area. During the Age of Discovery, it was known for ...
water bodies during the last melting of Ice Age
An ice age is a long period of reduction in the temperature of Earth's surface and atmosphere, resulting in the presence or expansion of continental and polar ice sheets and alpine glaciers. Earth's climate alternates between ice ages, and g ...
glacier
A glacier (; or ) is a persistent body of dense ice, a form of rock, that is constantly moving downhill under its own weight. A glacier forms where the accumulation of snow exceeds its ablation over many years, often centuries. It acquires ...
s. Some of the protactinium-related dating variations rely on analysis of the relative concentrations of several long-living members of the uranium decay chain – uranium, protactinium, and thorium, for example. These elements have 6, 5, and 4 valence electrons, thus favoring +6, +5, and +4 oxidation states respectively, and display different physical and chemical properties. Thorium and protactinium, but not uranium compounds, are poorly soluble in aqueous solutions and precipitate into sediments; the precipitation rate is faster for thorium than for protactinium. The concentration analysis for both protactinium-231 (half-life 32,760 years) and 230Th (half-life 75,380 years) improves measurement accuracy compared to when only one isotope is measured; this double-isotope method is also weakly sensitive to inhomogeneities in the spatial distribution of the isotopes and to variations in their precipitation rate.Articles "Protactinium" and "Protactinium-231 – thorium-230 dating" in Encyclopædia Britannica, 15th edition, 1995, p. 737
Precautions
Protactinium is both toxic and highly radioactive; thus, it is handled exclusively in a sealedglove box
A glovebox (or glove box) is a sealed container that is designed to allow one to manipulate objects where a separate atmosphere is desired. Built into the sides of the glovebox are gloves arranged in such a way that the user can place their hand ...
. Its major isotope 231Pa has a specific activity
Specific activity (symbol ''a'') is the activity per unit mass of a radionuclide and is a physical property of that radionuclide.
It is usually given in units of becquerel per kilogram (Bq/kg), but another commonly used unit of specific activi ...
of per gram and primarily emits alpha-particles with an energy of 5 MeV, which can be stopped by a thin layer of any material. However, it slowly decays, with a half-life of 32,760 years, into 227Ac, which has a specific activity of per gram, emits both alpha and beta radiation, and has a much shorter half-life of 22 years. 227Ac, in turn, decays into lighter isotopes with even shorter half-lives and much greater specific activities (SA).
As protactinium is present in small amounts in most natural products and materials, it is ingested with food or water and inhaled with air. Only about 0.05% of ingested protactinium is absorbed into the blood and the remainder is excreted. From the blood, about 40% of the protactinium
deposits in the bones, about 15% goes to the liver, 2% to the kidneys, and the rest leaves the body. The biological half-life of protactinium is about 50 years in the bones, whereas its biological half-life in other organs has a fast and slow component. For example, 70% of the protactinium in the liver has a biological half-life of 10 days, and the remaining 30% for 60 days. The corresponding values for kidneys are 20% (10 days) and 80% (60 days). In each affected organ, protactinium promotes cancer via its radioactivity. The maximum safe dose of Pa in the human body is , which corresponds to 0.5 micrograms of 231Pa. The maximum allowed concentrations of 231Pa in the air in Germany is .
See also
*Ada Hitchins
Ada Florence Remfry Hitchins (26 June 1891 – 4 January 1972) was the principal research assistant of British chemist Frederick Soddy, who won the Nobel prize in 1921 for work on radioactive elements and the theory of isotopes. Hitchins isola ...
, who helped Soddy in discovering the element protactinium
Notes
References
Bibliography
*External links
Protactinium
at ''
The Periodic Table of Videos
''Periodic Videos'' (also known as ''The Periodic Table of Videos'') is a video project and YouTube channel on chemistry. It consists of a series of videos about chemical elements and the periodic table, with additional videos on other topics i ...
'' (University of Nottingham)
{{Good article
Chemical elements
Chemical elements with body-centered tetragonal structure
Actinides
Chemical elements predicted by Dmitri Mendeleev