|
Isotopes Of Actinium
Actinium (89Ac) has no stable isotopes and no characteristic terrestrial isotopic composition, thus a standard atomic weight cannot be given. There are 34 known isotopes, from 203Ac to 236Ac, and 7 isomers. Three isotopes are found in nature, 225Ac, 227Ac and 228Ac, as intermediate decay products of, respectively, 237Np, 235U, and 232Th. 228Ac and 225Ac are extremely rare, so almost all natural actinium is 227Ac. The most stable isotopes are 227Ac with a half-life of 21.772 years, 225Ac with a half-life of 10.0 days, and 226Ac with a half-life of 29.37 hours. All other isotopes have half-lives under 10 hours, and most under a minute. The shortest-lived known isotope is 217Ac with a half-life of 69 ns. Purified 227Ac comes into equilibrium with its decay products (227Th and 223Fr) after 185 days. List of isotopes , -id=Actinium-203 , 203Ac , , style="text-align:right" , 89 , style="text-align:right" , 114 , , , α , 199Fr , (1/2+) , , -id=Actinium-204 , ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Actinium
Actinium is a chemical element; it has chemical symbol, symbol Ac and atomic number 89. It was discovered by Friedrich Oskar Giesel in 1902, who gave it the name ''emanium''; the element got its name by being wrongly identified with a substance André-Louis Debierne found in 1899 and called actinium. The actinide series, a set of 15 elements between actinium and lawrencium in the periodic table, are named for actinium. Together with polonium, radium, and radon, actinium was one of the first Primordial element, non-primordial radioactive elements to be discovered. A soft, silvery-white radioactive metal, actinium reacts rapidly with oxygen and moisture in air forming a white coating of actinium oxide that prevents further oxidation. As with most lanthanides and many actinides, actinium assumes oxidation state +3 in nearly all its chemical compounds. Actinium is found only in traces in uranium and thorium ores as the isotope 227Ac, which decays with a half-life of 21.772 years, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decay Product
In nuclear physics, a decay product (also known as a daughter product, daughter isotope, radio-daughter, or daughter nuclide) is the remaining nuclide left over from radioactive decay. Radioactive decay often proceeds via a sequence of steps ( decay chain). For example, 238U decays to 234Th which decays to 234mPa which decays, and so on, to 206Pb (which is stable): : \ce \overbrace^\ce left, upThe decay chain from lead-212 down to lead-208, showing the intermediate decay products In this example: * 234Th, 234mPa,...,206Pb are the decay products of 238U. * 234Th is the daughter of the parent 238U. * 234mPa (234 metastable) is the granddaughter of 238U. These might also be referred to as the daughter products of 238U. (''Depleted Uranium'' — authors: Naomi H. Harley, Ernest C. Foulkes, Lee H. Hilb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
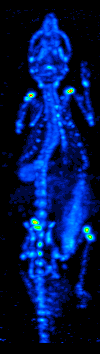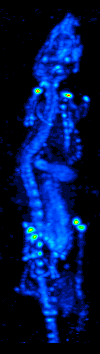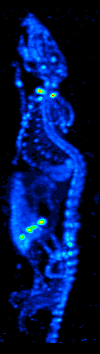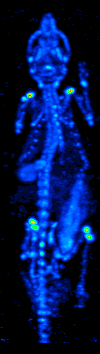
SPECT
Single-photon emission computed tomography (SPECT, or less commonly, SPET) is a nuclear medicine tomographic imaging technique using gamma rays. It is very similar to conventional nuclear medicine planar imaging using a gamma camera (that is, scintigraphy), but is able to provide true 3D information. This information is typically presented as cross-sectional slices through the patient, but can be freely reformatted or manipulated as required. The technique needs delivery of a gamma-emitting radioisotope (a radionuclide) into the patient, normally through injection into the bloodstream. On occasion, the radioisotope is a simple soluble dissolved ion, such as an isotope of gallium(III). Usually, however, a marker radioisotope is attached to a specific ligand to create a radioligand, whose properties bind it to certain types of tissues. This marriage allows the combination of ligand and radiopharmaceutical to be carried and bound to a place of interest in the body, where th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Capture
Electron capture (K-electron capture, also K-capture, or L-electron capture, L-capture) is a process in which the proton-rich nucleus of an electrically neutral atom absorbs an inner atomic electron, usually from the K or L electron shells. This process thereby changes a nuclear proton to a neutron and simultaneously causes the emission of an electron neutrino. : : or when written as a nuclear reaction equation, ^_e + ^_p -> ^_n + ^_ ν_e Since this single emitted neutrino carries the entire decay energy, it has this single characteristic energy. Similarly, the momentum of the neutrino emission causes the daughter atom to recoil with a single characteristic momentum. The resulting daughter nuclide, if it is in an excited state, then transitions to its ground state. Usually, a gamma ray is emitted during this transition, but nuclear de-excitation may also take place by internal conversion. Following capture of an inner electron from the atom, an outer elect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta Decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which an atomic nucleus emits a beta particle (fast energetic electron or positron), transforming into an isobar of that nuclide. For example, beta decay of a neutron transforms it into a proton by the emission of an electron accompanied by an antineutrino; or, conversely a proton is converted into a neutron by the emission of a positron with a neutrino in what is called ''positron emission''. Neither the beta particle nor its associated (anti-)neutrino exist within the nucleus prior to beta decay, but are created in the decay process. By this process, unstable atoms obtain a more stable ratio of protons to neutrons. The probability of a nuclide decaying due to beta and other forms of decay is determined by its nuclear binding energy. The binding energies of all existing nuclides form what is called the nuclear band or valley of stability. For either electron or positron emission to be energeticall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Breeder Reactors
A breeder reactor is a nuclear reactor that generates more fissile material than it consumes. These reactors can be fueled with more-commonly available isotopes of uranium and thorium, such as uranium-238 and thorium-232, as opposed to the rare uranium-235 which is used in conventional reactors. These materials are called fertile materials since they can be bred into fuel by these breeder reactors. Breeder reactors achieve this because their neutron economy is high enough to create more fissile fuel than they use. These extra neutrons are absorbed by the fertile material that is loaded into the reactor along with fissile fuel. This irradiated fertile material in turn transmutes into fissile material which can undergo fission reactions. Breeders were at first found attractive because they made more complete use of uranium fuel than light-water reactors, but interest declined after the 1960s as more uranium reserves were foundHelmreich, J. E. ''Gathering Rare Ores: The D ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uranium-233
Uranium-233 ( or U-233) is a fissile isotope of uranium that is bred from thorium-232 as part of the thorium fuel cycle. Uranium-233 was investigated for use in nuclear weapons and as a Nuclear fuel, reactor fuel. It has been used successfully in experimental nuclear reactors and has been proposed for much wider use as a nuclear fuel. It has a half-life of 160,000 years. Uranium-233 is produced by the neutron irradiation of thorium-232. When thorium-232 absorbs a neutron, it becomes thorium-233, which has a half-life of only 22 minutes. Thorium-233 decays into protactinium-233 through beta decay. Protactinium-233 has a half-life of 27 days and beta decays into uranium-233; some proposed molten salt reactor designs attempt to physically isolate the protactinium from further neutron capture before beta decay can occur, to maintain the neutron economy (if it misses the 233U window, the next fissile target is 235U, meaning a total of 4 neutrons needed to trigger fission). ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopes Of Thallium
Thallium (81Tl) has 42 isotopes with atomic masses that range from 176 to 217. 203Tl and 205Tl are the only stable isotopes and 204Tl is the most stable radioisotope with a half-life of 3.78 years. 207Tl, with a half-life of 4.77 minutes, has the longest half-life of naturally occurring Tl radioisotopes. All isotopes of thallium are either radioactive or observationally stable, meaning that they are predicted to be radioactive but no actual decay has been observed. Thallium-202 (half-life 12.23 days) can be made in a cyclotron while thallium-204 (half-life 3.78 years) is made by the neutron activation of stable thallium in a nuclear reactor. In the fully ionized state, the isotope 205Tl81+ becomes beta-radioactive, undergoing bound-state β− decay to 205Pb81+ with a half-life of days, but 203Tl remains stable. 205Tl is the decay product of bismuth-209, an isotope that was once thought to be stable but is now known to undergo alpha decay with an extremely long half-life of 2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bismuth-209
Bismuth-209 (Bi) is an isotope of bismuth, with the longest known half-life of any radioisotope that undergoes α-decay (alpha decay). It has 83 protons and a magic number of 126 neutrons, and an atomic mass of 208.9803987 amu (atomic mass units). Primordial bismuth consists entirely of this isotope. Decay properties Bismuth-209 was long thought to have the heaviest stable nucleus of any element, but in 2003, a research team at the Institut d’Astrophysique Spatiale in Orsay, France, discovered that Bi undergoes alpha decay with a half-life of 20.1 exayears (2.01×10, or 20.1 quintillion years), over 10 times longer than the estimated age of the universe. The heaviest nucleus considered to be stable is now lead-208 and the heaviest stable monoisotopic element is gold ( gold-197). Theory had previously predicted a half-life of 4.6 years. It had been suspected to be radioactive for a long time. The decay produces a 3.14 MeV alpha particle plus thallium-205. Bismuth-209 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopes Of Bismuth
Bismuth (83Bi) has 41 known isotopes, ranging from 184Bi to 224Bi. Bismuth has no stable isotopes, but does have one very long-lived isotope; thus, the standard atomic weight can be given as . Although bismuth-209 is now known to be radioactive, it has classically been considered to be a stable isotope because it has a half-life of approximately 2.01×1019 years, which is more than a billion times the age of the universe. Besides 209Bi, the most stable bismuth radioisotopes are 210mBi with a half-life of 3.04 million years, 208Bi with a half-life of 368,000 years and 207Bi, with a half-life of 32.9 years, none of which occur in nature. All other isotopes have half-lives under 1 year, most under a day. Of naturally occurring radioisotopes, the most stable is radiogenic 210Bi with a half-life of 5.012 days. 210mBi is unusual for being a nuclear isomer with a half-life multiple orders of magnitude longer than that of the ground state. List of isotopes , -id=Bismuth-184 , 184B ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Astatine-217
Astatine (85At) has 41 known isotopes, all of which are radioactive; their mass numbers range from 188 to 229 (though 189At is undiscovered). There are also 24 known metastable excited states. The longest-lived isotope is 210At, which has a half-life of 8.1 hours; the longest-lived isotope existing in naturally occurring decay chains is 219At with a half-life of 56 seconds. List of isotopes , -id=Astatine-188 , rowspan=2, 188At , rowspan=2 style="text-align:right" , 85 , rowspan=2 style="text-align:right" , 103 , rowspan=2, , rowspan=2, , α (~50%) , 184Bi , rowspan=2, , rowspan=2, , - , p (~50%) , 187Po , -id=Astatine-190 , 190At , style="text-align:right" , 85 , style="text-align:right" , 105 , , , α , 186Bi , (10−) , , -id=Astatine-191 , 191At , style="text-align:right" , 85 , style="text-align:right" , 106 , , , α , 187Bi , (1/2+) , , -id=Astatine-191m , style="text-indent:1em" , 191mAt , colspan="3" style="text-indent:2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Francium-221
Francium (87Fr) has no stable isotopes, thus a standard atomic weight cannot be given. Its most stable isotope is 223Fr with a half-life of 22 minutes, occurring in trace quantities in nature as an intermediate decay product of 235U. Of elements whose most stable isotopes have been identified with certainty, francium is the most unstable. All elements with atomic number of 106 (seaborgium) or greater have most-stable-known isotopes shorter than that of francium, but as those elements have only a relatively small number of isotopes discovered, the possibility remains that undiscovered isotopes of these elements may have longer half-lives. List of isotopes , -id=Francium-197 , 197Fr , , style="text-align:right" , 87 , style="text-align:right" , 110 , 197.01101(6) , 2.3(19) ms , α , 193At , (7/2−) , , -id=Francium-198 , 198Fr , , style="text-align:right" , 87 , style="text-align:right" , 111 , 198.01028(3) , 15(3) ms , α , 194At , 3+# , , -id=Fr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |