|
Uranocene
Uranocene, U(C8H8)2, is an organouranium compound composed of a uranium atom sandwiched between two cyclooctatetraene, cyclooctatetraenide rings. It was one of the first Organoactinide chemistry, organoactinide compounds to be synthesized. It is a green air-sensitive solid that dissolves in organic solvents. Uranocene, a member of the "actinocenes," a group of metallocenes incorporating Chemical element, elements from the actinide series. It is the most studied bisCyclooctatetraene, [8]annulene-metal system, although it has no known practical applications. Synthesis, structure and bonding Uranocene was first described in 1968 by the group of Andrew Streitwieser, when it was prepared by the reaction of dipotassium cyclooctatetraenide and uranium tetrachloride in THF at 0°C: : Uranocene is highly reactive toward oxygen, being pyrophoricity, pyrophoric in air but stable to hydrolysis. The x-ray crystal structure of uranocene was first elucidated by the group of Ken Raymond. Conside ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Actinocenes
Actinocenes are a family of organoactinide compounds consisting of metallocenes containing elements from the actinide series. They typically have a sandwich structure with two dianionic cyclooctatetraenyl ligands (COT2−, which is ) bound to an actinide-metal center (An) in the oxidation state IV, resulting in the general formula An(C8H8)2. Characterised actinocenes The most studied actinocene is uranocene, U(C8H8)2, which in 1968 was the first member of this family to be synthesised and is still viewed as the archetypal example. Other actinocenes that have been synthesised are protactinocene (Pa(C8H8)2), thorocene (Th(C8H8)2), neptunocene (Np(C8H8)2), and plutonocene (Pu(C8H8)2). Especially the latter two, neptunocene and plutonocene, have not been extensively studied experimentally since the 1980s because of the radiation hazard they pose. Berkelocene (with a modified COT ligand) was synthesised in 2025, the first actinocene with a new actinide in over 50 years. Bonding ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
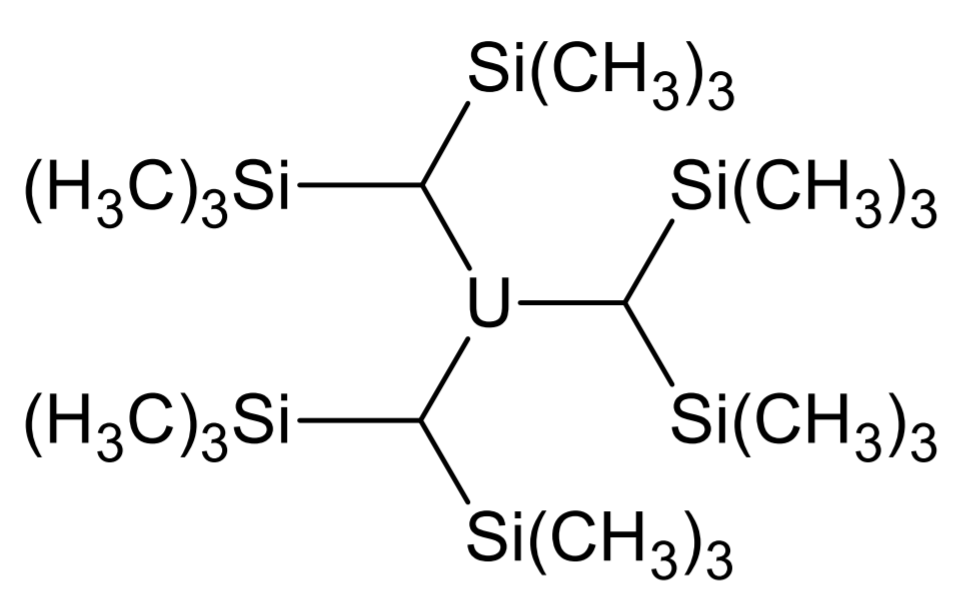
Organouranium Compound
Organouranium chemistry is the science exploring the properties, structure, and reactivity of organouranium compounds, which are organometallic compounds containing a carbon to uranium chemical bond. The field is of some importance to the nuclear industry and of theoretical interest in organometallic chemistry. History The development of organouranium compounds started in World War II when the Manhattan Project required volatile uranium compounds for 235U/238U isotope separation. For example, Henry Gilman attempted to synthesize compounds like tetramethyluranium, and others worked on uranium metal carbonyls, but none of the efforts met success due to organouranium instability. After the discovery of ferrocene in 1951, Todd Reynolds and Geoffrey Wilkinson in 1956 synthesized the uranium metallocene Cp3UCl from sodium cyclopentadienide and uranium tetrachloride as a stable but extremely air-sensitive compound. In it, the U-Cl bond is an ionic bond, while the bonds with the three ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organoactinide Chemistry
Organoactinide chemistry is the science exploring the properties, structure, and reactivity of organoactinide compounds, which are organometallic compounds containing a carbon to actinide chemical bond. Like most organometallic compounds, the organoactinides are air sensitive and need to be handled using the Air-free technique, appropriate methods. Organometallic complexes with σ-bonding Most common organoactinide complexes involve Pi bond, π-bonding with ligands such as cyclopentadienyl, but there are a few exceptions with Sigma bond, σ-bonding, namely in thorium and uranium chemistry as these are the most easily handleable elements of this group. Alkyl and aryl compounds Attempts to synthesize uranium alkyls were first made during the Manhattan project by Henry Gilman, inspired by the volatility of main group organometallics. However he noticed that these compounds tend to be highly unstable. Marks and Seyam attempted to synthesize them from Uranium tetrachloride, UCl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclooctatetraene
1,3,5,7-Cyclooctatetraene (COT) is an unsaturated derivative of cyclooctane, with the formula C8H8. It is also known as nnulene. This polyunsaturated hydrocarbon is a colorless to light yellow flammable liquid at room temperature. Because of its stoichiometric relationship to benzene, COT has been the subject of much research and some controversy. Unlike benzene, C6H6, cyclooctatetraene, C8H8, is not aromatic, although its dianion, ( cyclooctatetraenide), is. Its reactivity is characteristic of an ordinary polyene, i.e. it undergoes addition reactions. Benzene, by contrast, characteristically undergoes substitution reactions, not additions. History 1,3,5,7-Cyclooctatetraene was initially synthesized by Richard Willstätter in Munich in 1905 using pseudopelletierine as the starting material and the Hofmann elimination as the key transformation: : Willstätter noted that the compound did not exhibit the expected aromaticity. Between 1939 and 1943, chemists throughout ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sandwich Compound
In organometallic chemistry, a sandwich compound is a chemical compound featuring a metal bound by hapticity, haptic, covalent bonds to two arene compound, arene (ring) ligands. The arenes have the formula , substituted derivatives (for example ) and heterocycle, heterocyclic derivatives (for example ). Because the metal is usually situated between the two rings, it is said to be "sandwiched". A special class of sandwich complexes are the metallocenes. The term ''sandwich compound'' was introduced in organometallic nomenclature in 1956 in a report by J. D. Dunitz, L. E. Orgel and R. A. Rich, who confirmed the structure of ferrocene by X-ray crystallography. The correct structure, in which the molecule features an iron atom ''sandwiched'' between two parallel cyclopentadienyl rings, had been proposed several years previously by Robert Burns Woodward and, separately, by Ernst Otto Fischer. The structure helped explain puzzles about ferrocene's conformational isomer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclooctatetraenide Anion
In chemistry, the cyclooctatetraenide anion or cyclooctatetraenide, more precisely cyclooctatetraenediide, is an aromatic species with a formula of 8H8sup>2− and abbreviated as COT2−. It is the dianion of cyclooctatetraene. Salts of the cyclooctatetraenide anion can be stable, e.g., dipotassium cyclooctatetraenide or disodium cyclooctatetraenide. More complex coordination compounds are known as cyclooctatetraenide complexes, such as the actinocenes. The structure is a planar symmetric octagon stabilized by resonance, meaning each atom bears a charge of −. The length of the bond between carbon atoms is 1.432 Å. There are 10 π electrons. The structure can serve as a ligand with various metals. List of salts See also * Tropylium ion *Cyclopentadienyl anion Sodium cyclopentadienide is an organosodium compound with the formula C5H5Na. The compound is often abbreviated as NaCp, where Cp− is the cyclopentadienide anion. Sodium cyclopentadienide is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ken Raymond
Kenneth Norman Raymond (born January 7, 1942) is a bioinorganic and coordination chemist. He is Chancellor's Professor of Chemistry at the University of California, Berkeley, Professor of the Graduate School, the Director of the Seaborg Center in the Chemical Sciences Division at Lawrence Berkeley National Laboratory, and the President and Chairman of Lumiphore. Biography Early life and education Raymond was born on January 7, 1942, in Astoria, Oregon, and was raised in various towns in Oregon. After graduating from Clackamas High School in 1959, he spent a year in Germany where he worked as a test-driver for Volkswagen and developed a taste for German culture. He then attended Reed College in Portland, Oregon, where he majored in Chemistry and earned a Bachelor of Arts in 1964. Raymond then attended Northwestern University where he studied coordination chemistry and crystallography under Fred Basolo and also worked closely with James A. Ibers, earning his Ph.D. degree in 1968 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hapticity
In coordination chemistry, hapticity is the coordination complex, coordination of a ligand to a metal center via an uninterrupted and contiguous series of atoms. The hapticity of a ligand is described with the Greek letter eta (letter), η ('eta'). For example, η2 describes a ligand that coordinates through 2 contiguous atoms. In general the η-notation only applies when multiple atoms are coordinated (otherwise the denticity, κ-notation is used). In addition, if the ligand coordinates through multiple atoms that are contiguous then this is considered denticity (not hapticity), and the κ-notation is used once again. When naming complexes care should be taken not to confuse η with mu (letter), μ ('mu'), which relates to bridging ligands. History The need for additional nomenclature for organometallic compounds became apparent in the mid-1950s when Dunitz, Leslie Orgel, Orgel, and Rich described the structure of the "sandwich compound, sandwich complex" ferrocene by X-ray ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dipotassium Cyclooctatetraenide
Dipotassium cyclooctatetraenide, sometimes abbreviated K2COT, is an organopotassium compound with the formula K2C8H8. It is a brown solid that is used as a precursor to cyclooctatetraenide complexes, such as uranocene (U(C8H8)2). Analogs of K2C8H8 are known with ring substituents, with different alkali metals, and with various complexants. Preparation and structure Potassium cyclooctatetraenide is formed by the reaction of cyclooctatetraene with potassium metal: :2 K + C8H8 → K2C8H8 The reaction entails 2-electron reduction of the polyene and is accompanied by a color change from colorless to brown. The structure of K2(diglyme)C8H8 has been characterized by X-ray crystallography of the derivatives with diglyme Diglyme, or bis(2-methoxyethyl) ether, is an organic compound with the chemical formula . It is a colorless liquid with a slight ether-like odor. It is a solvent with a high boiling point. It is the dimethyl ether of diethylene glycol. The name '' ... complex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Bond
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds or through the sharing of electrons as in covalent bonds, or some combination of these effects. Chemical bonds are described as having different strengths: there are "strong bonds" or "primary bonds" such as covalent, ionic and metallic bonds, and "weak bonds" or "secondary bonds" such as dipole–dipole interactions, the London dispersion force, and hydrogen bonding. Since opposite electric charges attract, the negatively charged electrons surrounding the nucleus and the positively charged protons within a nucleus attract each other. Electrons shared between two nuclei will be attracted to both of them. "Constructive quantum mechanical wavefunction interference" stabilizes the paired nuclei (see Theories of chemical bonding). Bonded nuclei maintain an optima ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |