|
Radiolysis
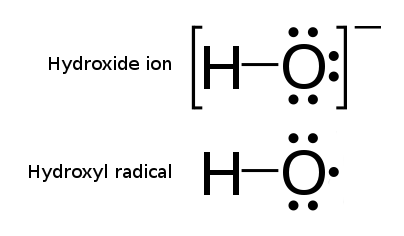
Radiolysis is the dissociation of molecules by ionizing radiation. It is the cleavage of one or several chemical bonds resulting from exposure to high-energy flux. The radiation in this context is associated with ionizing radiation; radiolysis is therefore distinguished from, for example, photolysis of the Cl2 molecule into two Cl-radicals, where (ultraviolet or visible spectrum) light is used. For example, water dissociates under alpha radiation into a hydrogen radical and a hydroxyl radical, unlike ionization of water which produces a hydrogen ion and a hydroxide ion. The chemistry of concentrated solutions under ionizing radiation is extremely complex. Radiolysis can locally modify redox conditions, and therefore the speciation and the solubility of the compounds. Water decomposition Of all the radiation-based chemical reactions that have been studied, the most important is the decomposition of water. When exposed to radiation, water undergoes a breakdown sequence into h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxyl Radical
The hydroxyl radical is the diatomic molecule . The hydroxyl radical is very stable as a dilute gas, but it decays very rapidly in the condensed phase. It is pervasive in some situations. Most notably the hydroxyl radicals are produced from the decomposition of hydroperoxides (ROOH) or, in atmospheric chemistry, by the reaction of excited atomic oxygen with water. It is also important in the field of radiation chemistry, since it leads to the formation of hydrogen peroxide and oxygen, which can enhance corrosion and SCC in coolant systems subjected to radioactive environments. In organic synthesis, hydroxyl radicals are most commonly generated by photolysis of 1-hydroxy-2(1''H'')-pyridinethione. Notation The unpaired electron of the hydroxyl radical is officially represented by a middle dot, •, beside the O. Biology Hydroxyl radicals can occasionally be produced as a byproduct of immune action. Macrophages and microglia most frequently generate this compound wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ionizing Radiation
Ionizing radiation (or ionising radiation), including nuclear radiation, consists of subatomic particles or electromagnetic waves that have sufficient energy to ionize atoms or molecules by detaching electrons from them. Some particles can travel up to 99% of the speed of light, and the electromagnetic waves are on the high-energy portion of the electromagnetic spectrum. Gamma rays, X-rays, and the higher energy ultraviolet part of the electromagnetic spectrum are ionizing radiation, whereas the lower energy ultraviolet, visible light, nearly all types of laser light, infrared, microwaves, and radio waves are non-ionizing radiation. The boundary between ionizing and non-ionizing radiation in the ultraviolet area is not sharply defined, as different molecules and atoms ionize at different energies. The energy of ionizing radiation starts between 10 electronvolts (eV) and 33 eV. Typical ionizing subatomic particles include alpha particles, beta particle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dissociation (chemistry)
Dissociation in chemistry is a general process in which molecules (or ionic compounds such as salts, or complexes) separate or split into other things such as atoms, ions, or radicals, usually in a reversible manner. For instance, when an acid dissolves in water, a covalent bond between an electronegative atom and a hydrogen atom is broken by heterolytic fission, which gives a proton (H+) and a negative ion. Dissociation is the opposite of association or recombination. Dissociation constant For reversible dissociations in a chemical equilibrium :AB A + B the dissociation constant ''K''d is the ratio of dissociated to undissociated compound :K_d = \mathrm where the brackets denote the equilibrium concentrations of the species. Dissociation degree The dissociation degree \alpha is the fraction of original solute molecules that have dissociated. It is usually indicated by the Greek symbol α. More accurately, degree of dissociation refers to the amount of solute dissoci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solubility
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution. The extent of the solubility of a substance in a specific solvent is generally measured as the concentration of the solute in a saturated solution, one in which no more solute can be dissolved. At this point, the two substances are said to be at the solubility equilibrium. For some solutes and solvents, there may be no such limit, in which case the two substances are said to be " miscible in all proportions" (or just "miscible"). The solute can be a solid, a liquid, or a gas, while the solvent is usually solid or liquid. Both may be pure substances, or may themselves be solutions. Gases are always miscible in all proportions, except in very extreme situations,J. de Swaan Arons and G. A. M. Diepen (1966): "Gas—Gas Equilibria". ''Journal of Chemical Phys ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Borate
Sodium borate is a generic name for any salt of sodium with an anion consisting of boron and oxygen, and possibly hydrogen, or any hydrate thereof. It can be seen as a hydrated sodium salt of the appropriate boroxy acid, although the latter may not be a stable compound. Many sodium borates have important industrial and household applications; the best known being borax, = . The ternary phase diagram of the –– phase diagram in the 0–100 °C temperature range contains 13 unique hydrated crystalline sodium borates, including five important industrial products. Sodium borates, as well as boroxy acids, are often described as mixtures = , with ''x'', ''y'', and ''z'' chosen to fit the elemental formula, or a multiple thereof. Thus, for example, borax would be , and boric acid would be = . The elemental formula was often intrepreted as a ''z''-hydrate of an "anhydrous" salt without any hydrogen, namely . However, later research uncovered that many borates have hydroxyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radioactive Waste
Radioactive waste is a type of hazardous waste that contains radioactive material. Radioactive waste is a result of many activities, including nuclear medicine, nuclear research, nuclear power generation, rare-earth mining, and nuclear weapons reprocessing. The storage and disposal of radioactive waste is regulated by government agencies in order to protect human health and the environment. Radioactive waste is broadly classified into low-level waste (LLW), such as paper, rags, tools, clothing, which contain small amounts of mostly short-lived radioactivity, intermediate-level waste (ILW), which contains higher amounts of radioactivity and requires some shielding, and high-level waste (HLW), which is highly radioactive and hot due to decay heat, so requires cooling and shielding. In nuclear reprocessing plants about 96% of spent nuclear fuel is recycled back into uranium-based and mixed-oxide (MOX) fuels. The residual 4% is minor actinides and fission products the latte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electronvolt
In physics, an electronvolt (symbol eV, also written electron-volt and electron volt) is the measure of an amount of kinetic energy gained by a single electron accelerating from rest through an electric potential difference of one volt in vacuum. When used as a unit of energy, the numerical value of 1 eV in joules (symbol J) is equivalent to the numerical value of the charge of an electron in coulombs (symbol C). Under the 2019 redefinition of the SI base units, this sets 1 eV equal to the exact value Historically, the electronvolt was devised as a standard unit of measure through its usefulness in electrostatic particle accelerator sciences, because a particle with electric charge ''q'' gains an energy after passing through a voltage of ''V.'' Since ''q'' must be an integer multiple of the elementary charge ''e'' for any isolated particle, the gained energy in units of electronvolts conveniently equals that integer times the voltage. It is a common unit of ene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Reprocessing
Nuclear reprocessing is the chemical separation of fission products and actinides from spent nuclear fuel. Originally, reprocessing was used solely to extract plutonium for producing nuclear weapons. With commercialization of nuclear power, the reprocessed plutonium was recycled back into MOX nuclear fuel for thermal reactors. The reprocessed uranium, also known as the spent fuel material, can in principle also be re-used as fuel, but that is only economical when uranium supply is low and prices are high. A breeder reactor is not restricted to using recycled plutonium and uranium. It can employ all the actinides, closing the nuclear fuel cycle and potentially multiplying the energy extracted from natural uranium by about 60 times. Reprocessing must be highly controlled and carefully executed in advanced facilities by highly specialized personnel. Fuel bundles which arrive at the sites from nuclear power plants (after having cooled down for several years) are completely ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spent Nuclear Fuel
Spent nuclear fuel, occasionally called used nuclear fuel, is nuclear fuel that has been irradiated in a nuclear reactor (usually at a nuclear power plant). It is no longer useful in sustaining a nuclear reaction in an ordinary thermal reactor and depending on its point along the nuclear fuel cycle, it may have considerably different isotopic constituents. The term "fuel" is slightly confusing, as it implies a combustion of some type, which does not occur in a nuclear power plant. Nevertheless, this term is generally accepted. Nature of spent fuel Nanomaterial properties In the oxide fuel, intense temperature gradients exist that cause fission products to migrate. The zirconium tends to move to the centre of the fuel pellet where the temperature is highest, while the lower-boiling fission products move to the edge of the pellet. The pellet is likely to contain many small bubble-like pores that form during use; the fission product xenon migrates to these voids. Some of this xen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Reactor
A nuclear reactor is a device used to initiate and control a fission nuclear chain reaction or nuclear fusion reactions. Nuclear reactors are used at nuclear power plants for electricity generation and in nuclear marine propulsion. Heat from nuclear fission is passed to a working fluid (water or gas), which in turn runs through steam turbines. These either drive a ship's propellers or turn electrical generators' shafts. Nuclear generated steam in principle can be used for industrial process heat or for district heating. Some reactors are used to produce isotopes for Nuclear medicine, medical and industrial radiography, industrial use, or for production of weapons-grade plutonium. , the International Atomic Energy Agency reports there are 422 nuclear power reactors and 223 nuclear research reactors in operation around the world. In the early era of nuclear reactors (1940s), a reactor was known as a nuclear pile or atomic pile (so-called because the graphite moderator blocks of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Corrosion
Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials (usually a metal) by chemical or electrochemical reaction with their environment. Corrosion engineering is the field dedicated to controlling and preventing corrosion. In the most common use of the word, this means electrochemical oxidation of metal in reaction with an oxidant such as oxygen, hydrogen or hydroxide. Rusting, the formation of iron oxides, is a well-known example of electrochemical corrosion. This type of damage typically produces oxide(s) or salt(s) of the original metal and results in a distinctive orange colouration. Corrosion can also occur in materials other than metals, such as ceramics or polymers, although in this context, the term "degradation" is more common. Corrosion degrades the useful properties of materials and structures including strength, appearance and permeability to liquids and gases. Many ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Light-water Reactor
The light-water reactor (LWR) is a type of thermal-neutron reactor that uses normal water, as opposed to heavy water, as both its coolant and neutron moderator; furthermore a solid form of fissile elements is used as fuel. Thermal-neutron reactors are the most common type of nuclear reactor, and light-water reactors are the most common type of thermal-neutron reactor. There are three varieties of light-water reactors: the pressurized water reactor (PWR), the boiling water reactor (BWR), and (most designs of) the supercritical water reactor (SCWR). History Early concepts and experiments After the discoveries of fission, moderation and of the theoretical possibility of a nuclear chain reaction, early experimental results rapidly showed that natural uranium could only undergo a sustained chain reaction using graphite or heavy water as a moderator. While the world's first reactors ( CP-1, X10 etc.) were successfully reaching criticality, uranium enrichment began to develop f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |