Carbenium ion on:
[Wikipedia]
[Google]
[Amazon]
The carbenium ion is a kind of positive ion with the structure RR′R″C+, that is, a



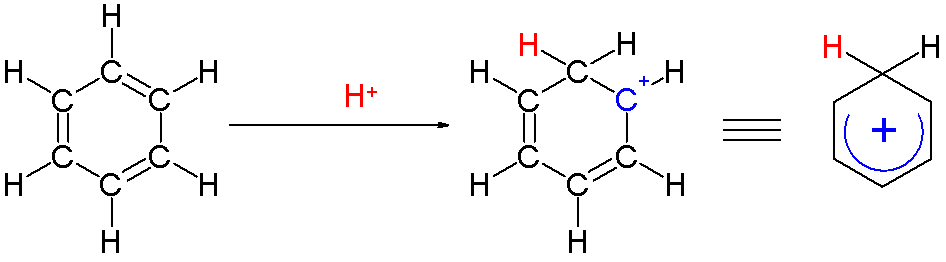 Two hydrogen atoms bonded to one carbon lie in a plane perpendicular to the benzene ring. The arenium ion is no longer an aromatic species; however it is relatively stable due to delocalization: the positive charge is delocalized over 5 carbon atoms. Also contributing to the stability of arenium ions is the energy gain resulting from the strong C-e bond (E = electrophile).
The smallest arenium ion is protonated
Two hydrogen atoms bonded to one carbon lie in a plane perpendicular to the benzene ring. The arenium ion is no longer an aromatic species; however it is relatively stable due to delocalization: the positive charge is delocalized over 5 carbon atoms. Also contributing to the stability of arenium ions is the energy gain resulting from the strong C-e bond (E = electrophile).
The smallest arenium ion is protonated
chemical species
Chemical species are a specific form of chemical substance or chemically identical molecular entities that have the same molecular energy level at a specified timescale. These entities are classified through bonding types and relative abundance of ...
with carbon atom having three covalent bonds, and it bears a +1 formal charge
In chemistry, a formal charge (F.C. or ), in the covalent view of chemical bonding, is the hypothetical charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between atoms, regardless of rela ...
. Carbenium ions are a major subset of carbocation
Carbocation is a general term for ions with a positively charged carbon atom. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further ...
s, which is a general term for diamagnetic carbon-based cations. In parallel with carbenium ions is another subset of carbocations, the carbonium ions with the formula R5+. In carbenium ions charge is localized. They are isoelectronic with monoborane
Borane is an inorganic compound with the chemical formula . Because it tends to dimerize or form adducts, borane is very rarely observed. It normally dimerizes to diborane in the absence of other chemicals. It can be observed directly as a c ...
s such as B(CH3)3.
Nomenclature
Reactivity
Carbenium ions are generally highly reactive due to having an incomplete octet of electrons; however, certain carbenium ions, such as the tropylium ion, are relatively stable due to the positive charge being delocalised between the carbon atoms.(It can even exist stably in aqueous solution.)Rearrangements
Carbenium ions sometimes rearrange readily. For example, when pentan-3-ol is heated with aqueous HCl, the initially formed 3-pentyl carbocation rearranges to a mixture of the 3-pentyl and 2-pentyl. These cations react with chloride ion to produce 3-chloropentane and 2-chloropentane in a ratio of approximately 1:2. Migration of an alkyl group to form a new carbocationic center is also observed. This often occurs withrate constant
In chemical kinetics, a reaction rate constant or reaction rate coefficient () is a proportionality constant which quantifies the rate and direction of a chemical reaction by relating it with the concentration of reactants.
For a reaction between ...
s in excess of 1010 s−1 at ambient temperature and still takes place rapidly (compared to the NMR timescale) at temperatures as low as −120 °C (''see Wagner-Meerwein shift''). In especially favorable cases like the 2-norbornyl cation, hydrogen shifts may still take place at rates fast enough to interfere with X-ray crystallography at . Typically, carbocations will rearrange to give a tertiary isomer. For instance, all isomers of rapidly rearrange to give the 1-methyl-1-cyclopentyl cation. This fact often complicates synthetic pathways. For example, when 3-pentanol is heated with aqueous HCl, the initially formed 3-pentyl carbocation rearranges to a statistical mixture of the 3-pentyl and 2-pentyl. These cations react with chloride ion to produce about one third 3-chloropentane and two thirds 2-chloropentane. The Friedel–Crafts alkylation suffers from this limitation; for this reason, the acylation
In chemistry, acylation is a broad class of chemical reactions in which an acyl group () is added to a substrate. The compound providing the acyl group is called the acylating agent. The substrate to be acylated and the product include the foll ...
(followed by Wolff–Kishner or Clemmensen reduction
Clemmensen reduction is a chemical reaction described as a Redox, reduction of ketones or aldehydes to alkanes using zinc Amalgam (chemistry), amalgam and concentrated hydrochloric acid (HCl). This reaction is named after Erik Christian Clemmensen ...
to give the alkylated product) is more frequently applied.
As electrophiles
Carbocations are susceptible to attack bynucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
s, like water, alcohols, carboxylates, azide, and halide ions, to form the addition product. Strongly basic nucleophiles, especially hindered ones, favor elimination over addition. Because even weak nucleophiles will react with carbocations, most can only be directly observed or isolated in non-nucleophilic media like superacid
In chemistry, a superacid (according to the original definition) is an acid with an acidity greater than that of 100% pure sulfuric acid (), which has a Hammett acidity function (''H''0) of −12. According to the modern definition, a superacid i ...
s.

Types of carbenium ions
Stability
The stability order of carbocations, from most stable to least stable as reflected by hydride ion affinity (HIA) values, are as follows (HIA values in kcal/mol in parentheses): Since carbenium ions can be highly reactive, a major consideration is their stability. The stability of carbenium ions correlates with the electron-donating properties of the substituents. Trialkylcarbenium ions, such as , are isolable as salts, but cannot. An analogous situation applies to triarylcarbenium ions: salts of triphenylcarbenium are readily isolable (seetrityl
Triphenylmethane or triphenyl methane (sometimes also known as Tritan), is the hydrocarbon with the chemical formula, formula (C6H5)3CH. This colorless solid is soluble in nonpolar organic solvents and not in water. Triphenylmethane is the basic sk ...
), and those with amine substituents so robust that they are used as dyes, e.g. crystal violet
Crystal violet or gentian violet, also known as methyl violet 10B or hexamethyl pararosaniline chloride, is a triphenylmethane, triarylmethane dye used as a histological stain and in Gram staining, Gram's method of classifying bacteria. Crystal ...
. Carbenium ions can also be stabilized by conjugation to double bonds giving allyl cations, which enjoy some resonance stabilization
In chemistry, resonance, also called mesomerism, is a way of describing bonding in certain molecules or polyatomic ions by the combination of several contributing structures (or ''forms'', also variously known as ''resonance structures'' or '' ...
. This situation is illustrated by the isolation of protonated benzene. Lone-pair bearing heteroatom
In chemistry, a heteroatom () is, strictly, any atom that is not carbon or hydrogen.
Organic chemistry
In practice, the term is mainly used more specifically to indicate that non-carbon atoms have replaced carbon in the backbone of the molecular ...
s also stabilize carbenium ions.Hansjörg Grützmacher, Christina M. Marchand (1997), "Heteroatom stabilized carbenium ions", ''Coord. Chem. Rev.'', 163, 287–344.
Alkylium ions
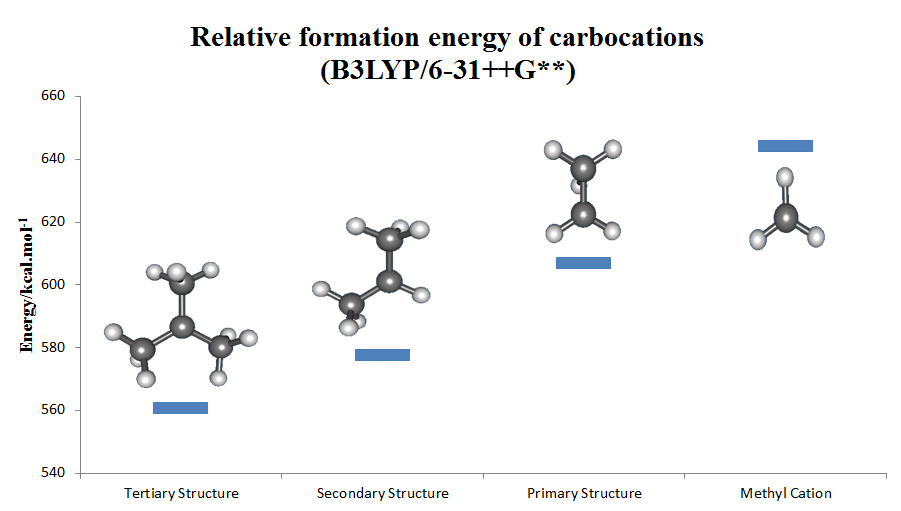
The stability of alkyl-substituted carbocations follows the order . This trend can be inferred by the hydride ion affinity values (231, 246, 273, and 312 kcal/mol for , , , and ). The effect of alkyl substitution is a strong one: *tertiary cations are stable and many are directly observable in superacid media. The stabilization by alkyl groups is explained byhyperconjugation
In organic chemistry, hyperconjugation (σ-conjugation or no-bond resonance) refers to the delocalization of electrons with the participation of bonds of primarily σ-character. Usually, hyperconjugation involves the interaction of the electron ...
. The donation of electron density from a β C-H or C-C bond into the unoccupied p orbital of the carbocation (a σCH/CC → p interaction) allows the positive charge to be delocalized.
*Secondary cations are usually transient. Only the isopropyl, ''s''-butyl, and cyclopentyl cations have been observed in solution.
*Primary carbocations in the solution phase, even as transient intermediates (the ethyl cation has been proposed for reactions in 99.9% sulfuric acid and in ), and methyl cation has only been unambiguously identified in the gas phase. In most, if not all cases, the ground state of alleged primary carbenium ions consist of bridged structures in which positive charge is shared by two or more carbon atoms and are better described as side-protonated alkenes, edge-protonated cyclopropanes, or corner-protonated cyclopropanes rather than true primary cations. The simple ethyl cation, has been demonstrated experimentally and computationally to be bridged and can be thought of as a symmetrically protonated ethylene molecule. The same is true for higher homologues like 1-propyl and 1-butyl cations. Neopentyl derivatives are thought to ionize with concomitant migration of a methyl group ( anchimeric assistance); thus, in most if not all cases, a discrete neopentyl cation is not believed to be involved.
Carbenium ions can be prepared directly from alkane
In organic chemistry, an alkane, or paraffin (a historical trivial name that also has other meanings), is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in whi ...
s by removing a hydride
In chemistry, a hydride is formally the anion of hydrogen (H−), a hydrogen ion with two electrons. In modern usage, this is typically only used for ionic bonds, but it is sometimes (and has been more frequently in the past) applied to all che ...
anion, , with a strong acid. For example, magic acid
Magic acid () is a superacid consisting of a mixture, most commonly in a 1:1 molar ratio, of fluorosulfuric acid () and antimony pentafluoride (). This conjugate Brønsted acid, Brønsted–Lewis acid, Lewis superacid system was developed in the 1 ...
, a mixture of antimony pentafluoride
Antimony pentafluoride is the inorganic compound with the formula Sb F5. This colorless, viscous liquid is a strong Lewis acid and a component of the superacid fluoroantimonic acid, formed upon mixing liquid HF with liquid SbF5 in 1:1 ratio. It ...
() and fluorosulfuric acid
Fluorosulfuric acid (IUPAC name: sulfurofluoridic acid) is the inorganic compound with the chemical formula . It is one of the strongest acids commercially available. It is a tetrahedral molecule and is closely related to sulfuric acid, , substitu ...
(), turns isobutane
Isobutane, also known as ''i''-butane, 2-methylpropane or methylpropane, is a chemical compound with molecular formula HC(CH3)3. It is an isomer of butane. Isobutane is a colorless, odorless gas.
It is the simplest alkane with a tertiary carbon a ...
into the trimethylcarbenium cation, .George A. Olah and Joachim Lukas (1967), "Stable Carbonium Ions. XLVII. Alkylcarbonium ion formation from alkanes via hydride (alkide) ion abstraction in fluorosulfonic acid-antimony pentafluoride-sulfuryl chlorofluoride solution". ''J. Am. Chem. Soc.'' 89 (18), 4739–4744
Extra stabilizing effects
A carbocation may be stabilized byresonance
Resonance is a phenomenon that occurs when an object or system is subjected to an external force or vibration whose frequency matches a resonant frequency (or resonance frequency) of the system, defined as a frequency that generates a maximu ...
by a carbon–carbon double bond or by the lone pair of a heteroatom
In chemistry, a heteroatom () is, strictly, any atom that is not carbon or hydrogen.
Organic chemistry
In practice, the term is mainly used more specifically to indicate that non-carbon atoms have replaced carbon in the backbone of the molecular ...
adjacent to the ionized carbon. The ''allyl
In organic chemistry, an allyl group is a substituent with the structural formula . It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . In 1844, Theodor Wertheim isolated a ...
'' cation and ''benzyl
In organic chemistry, benzyl is the substituent or molecular fragment possessing the structure . Benzyl features a benzene ring () attached to a methylene group ().
Nomenclature
In IUPAC nomenclature, the prefix benzyl refers to a substituent ...
'' cation are more stable than most other carbenium ions due to donation of electron density from π systems to the cationic center. The doubly- and triply-benzylic carbocations, diphenylcarbenium and triphenylcarbenium (trityl) cation, are particularly stable. For the same reasons, the partial p character of strained C–C bonds in cyclopropyl groups also allows for donation of electron density and stabilizes the ''cyclopropylmethyl'' (cyclopropylcarbinyl) cation.
Oxocarbenium and iminium
In organic chemistry, an iminium cation is a polyatomic ion with the general structure . They are common in synthetic chemistry and biology.
Structure
Iminium cations adopt alkene-like geometries: the central C=N unit is nearly coplanar with a ...
ions have important secondary canonical forms (resonance structures) in which carbon bears a positive charge. As such, they are carbocations according to the IUPAC definition although some chemists do not regard them to be "true" carbocations, as their most important resonance contributors carry the formal positive charge on an oxygen or nitrogen atom, respectively.

Aromatic carbenium ions
The tropylium ion is anaromatic
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated system, conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugati ...
species with the formula . Its name derives from the molecule tropine (itself named for the molecule atropine
Atropine is a tropane alkaloid and anticholinergic medication used to treat certain types of nerve agent and pesticide poisonings as well as some types of slow heart rate, and to decrease saliva production during surgery. It is typically give ...
). Salts of the tropylium cation can be stable, e.g. tropylium tetrafluoroborate. It can be made from cycloheptatriene
Cycloheptatriene (CHT) is an organic compound with the chemical formula, formula C7H8. It is a closed ring of seven carbon atoms joined by three double bonds (as the name implies) and four single bonds. This colourless liquid has been of recurring ...
(tropylidene) and bromine
Bromine is a chemical element; it has chemical symbol, symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between th ...
or phosphorus pentachloride
Phosphorus pentachloride is the chemical compound with the formula . It is one of the most important phosphorus chlorides/oxychlorides, others being and . finds use as a chlorinating reagent. It is a colourless, water-sensitive solid, althoug ...
.
It is a planar, cyclic, heptagon
In geometry, a heptagon or septagon is a seven-sided polygon or 7-gon.
The heptagon is sometimes referred to as the septagon, using ''Wikt:septa-, septa-'' (an elision of ''Wikt:septua-, septua-''), a Latin-derived numerical prefix, rather than ...
al ion; it also has 6 π-electrons (4''n'' + 2, where ''n'' = 1), which fulfills Hückel's rule
In organic chemistry, Hückel's rule predicts that a planar ring molecule will have aromatic properties if it has 4''n'' + 2 π-electrons, where ''n'' is a non-negative integer. The quantum mechanical basis for its formulation was f ...
of aromaticity. It can coordinate as a ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
to metal
A metal () is a material that, when polished or fractured, shows a lustrous appearance, and conducts electrical resistivity and conductivity, electricity and thermal conductivity, heat relatively well. These properties are all associated wit ...
atoms
Atoms are the basic particles of the chemical elements. An atom consists of a nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished from each other ...
.
The structure shown is a composite of seven resonance contributors in which each carbon carries part of the positive charge.
In 1891 G. Merling obtained a water-soluble salt from a reaction of cycloheptatriene and bromine. The structure was elucidated by Eggers Doering and Knox in 1954.
On the other hand, the antiaromatic cyclopentadienyl cation () is destabilized by some 40 kcal/mol.
Another aromatic carbenium ion is the cyclopropenyl or cyclopropenium ion, . Although less stable than the tropylium cation, this carbenium ion can also form salts at room temperature. Solutions of such salts were exhibit conventional spectroscopic and chemical properties. The cyclopropenium cation (), although somewhat destabilized by angle strain, is still clearly stabilized by aromaticity when compared to its open-chain analog, allyl cation.
These varying cation stabilities, depending on the number of π electrons in the ring system, can furthermore be crucial factors in reaction kinetics. The formation of an aromatic carbocation is much faster than the formation of an anti-aromatic or open-chain carbocation.

Arenium ions
An arenium ion is a cyclohexadienyl cation that appears as a reactive intermediate inelectrophilic aromatic substitution
Electrophilic aromatic substitution (SEAr) is an organic reaction in which an atom that is attached to an aromatic ring, aromatic system (usually hydrogen) is replaced by an electrophile. Some of the most important electrophilic aromatic substitut ...
. For historic reasons this complex is also called a ''Wheland intermediate'', or a ''σ-complex''.
: Two hydrogen atoms bonded to one carbon lie in a plane perpendicular to the benzene ring. The arenium ion is no longer an aromatic species; however it is relatively stable due to delocalization: the positive charge is delocalized over 5 carbon atoms. Also contributing to the stability of arenium ions is the energy gain resulting from the strong C-e bond (E = electrophile).
The smallest arenium ion is protonated
Two hydrogen atoms bonded to one carbon lie in a plane perpendicular to the benzene ring. The arenium ion is no longer an aromatic species; however it is relatively stable due to delocalization: the positive charge is delocalized over 5 carbon atoms. Also contributing to the stability of arenium ions is the energy gain resulting from the strong C-e bond (E = electrophile).
The smallest arenium ion is protonated benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hyd ...
, . The benzenium ion can be isolated as a stable compound when benzene is protonated by the carborane superacid, H(CB11H(CH3)5Br6). The benzenium salt is crystalline with thermal stability up to 150 °C. Bond length
In molecular geometry, bond length or bond distance is defined as the average distance between Atomic nucleus, nuclei of two chemical bond, bonded atoms in a molecule. It is a Transferability (chemistry), transferable property of a bond between at ...
s deduced from X-ray crystallography
X-ray crystallography is the experimental science of determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to Diffraction, diffract in specific directions. By measuring th ...
are consistent with a cyclohexadienyl cation structure.
Acylium ions
An acylium ion is a cation with the formula RCO+. The structure is described as R−C≡O+ or R−=O. It is an acyl carbocation, but the actual structure has the oxygen and carbon linked by a triple bond. Such species are common reactive intermediates, for example, in the Friedel−Crafts acylations also in many otherorganic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, mechanistic organ ...
s such as the Hayashi rearrangement. Salts containing acylium ions can be generated by removal of the halide from acyl halides:
:RCOCl + SbCl5 → RCO+
The C–O distance in these cations is near 1.1 ångström
The angstrom (; ) is a unit of length equal to m; that is, one ten- billionth of a metre, a hundred-millionth of a centimetre, 0.1 nanometre, or 100 picometres. The unit is named after the Swedish physicist Anders Jonas Ångström (1814� ...
s, even shorter than that in carbon monoxide
Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
. Acylium cations are characteristic fragments observed in EI- mass spectra of ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
s.
Vinyl and alkynyl carbenium ions
Based on hydride ion affinity, the parent vinyl cation is less stable than even a primary sp2-hybridized carbocation, while an α alkyl-substituted vinyl cation has a stability that is comparable to the latter. Hence, vinyl cations are relatively uncommon intermediates. They can be generated by the ionization of a vinyl electrophile, provided the leaving group is sufficiently good (e.g., , IPh, or ). They have been implicated as intermediates in some vinyl substitution reactions (designated as SN1(vinyl)) and as intermediates in the electrophilic addition reactions of arylalkynes. With the exception of the parent vinyl cation, which is believed to be a bridged species, and geometrically constrained cyclic vinyl cations, most vinyl cations take on sp hybridization and are linear. Aryl cations are less stable than vinyl cations due to the ring-enforced distortion to a nonlinear geometry and approximately sp2-character of the unoccupied orbital. Only in aryldiazonium salts is a good enough leaving group for the chemical generation of aryl cations. Alkynyl cations are extremely unstable, much less stable than even (hydride ion affinity 386 kcal/mol versus 312 kcal/mol for ) and cannot be generated by purely chemical means. They can, however, be generated radiochemically via thebeta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which an atomic nucleus emits a beta particle (fast energetic electron or positron), transforming into an isobar of that nuclide. For example, beta decay of a neutron ...
of tritium
Tritium () or hydrogen-3 (symbol T or H) is a rare and radioactive isotope of hydrogen with a half-life of ~12.33 years. The tritium nucleus (t, sometimes called a ''triton'') contains one proton and two neutrons, whereas the nucleus of the ...
:
:
Selected applications
122px Carbenium ions are so integrated into organic chemistry that a full inventory of their commercially useful reactions would be long. For example, catalytic cracking, a major step inpetroleum refining
An oil refinery or petroleum refinery is an industrial process plant where petroleum (crude oil) is transformed and refined into products such as gasoline (petrol), diesel fuel, asphalt base, fuel oils, heating oil, kerosene, liquefied petr ...
involves carbenium ion intermediates.
The alkylation Alkylation is a chemical reaction that entails transfer of an alkyl group. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effecting al ...
of benzene with alpha-olefin
In organic chemistry, terminal alkenes (alpha-olefins, α-olefins, or 1-alkenes) are a family of organic compounds which are alkenes (also known as olefins) with a chemical formula , distinguished by having a double bond at the primary, Alpha an ...
s to give linear alkylbenzene (LABs) illustrates the behaviour of secondary carbenium ions. The alkylation is initiated by strong acids. LABs are a key precursor to detergent
A detergent is a surfactant or a mixture of surfactants with Cleanliness, cleansing properties when in Concentration, dilute Solution (chemistry), solutions. There are a large variety of detergents. A common family is the alkylbenzene sulfonate ...
s.
Derivatives of the triphenylcarbenium are the triarylmethane dyes.
Acylium ions are intermediates in Friedel-Crafts acylations and Koch reactions.
See also
* Borenium ion * Nitrenium ionReferences
{{reflist Cations Reactive intermediates Carbocations