|
Antiaromatic
Antiaromaticity is a chemical property of a cyclic molecule with a π electron system that has higher energy, i.e., it is less stable due to the presence of 4n delocalised (π or lone pair) electrons in it, as opposed to aromaticity. Unlike aromatic compounds, which follow Hückel's rule ( ''n''+2π electrons) and are highly stable, antiaromatic compounds are highly unstable and highly reactive. To avoid the instability of antiaromaticity, molecules may change shape, becoming non-planar and therefore breaking some of the π interactions. In contrast to the diamagnetic ring current present in aromatic compounds, antiaromatic compounds have a paramagnetic ring current, which can be observed by NMR spectroscopy. Examples of antiaromatic compounds are pentalene (A), biphenylene (B), cyclopentadienyl cation (C). The prototypical example of antiaromaticity, cyclobutadiene, is the subject of debate, with some scientists arguing that antiaromaticity is not a major factor contributing t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aromaticity
In chemistry, aromaticity is a chemical property of cyclic (ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to saturated compounds having single bonds, and other geometric or connective non-cyclic arrangements with the same set of atoms. Aromatic rings are very stable and do not break apart easily. Organic compounds that are not aromatic are classified as aliphatic compounds—they might be cyclic, but only aromatic rings have enhanced stability. The term ''aromaticity'' with this meaning is historically related to the concept of having an aroma, but is a distinct property from that meaning. Since the most common aromatic compounds are derivatives of benzene (an aromatic hydrocarbon common in petroleum and its distillates), the word ''aromatic'' occasionally refers informally to benzene derivatives, and so it was first defined. Nevertheless, man ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclooctatetraene Smaller
1,3,5,7-Cyclooctatetraene (COT) is an unsaturated derivative of cyclooctane, with the formula C8H8. It is also known as nnulene. This polyunsaturated hydrocarbon is a colorless to light yellow flammable liquid at room temperature. Because of its stoichiometric relationship to benzene, COT has been the subject of much research and some controversy. Unlike benzene, C6H6, cyclooctatetraene, C8H8, is not aromatic, although its dianion, (cyclooctatetraenide), is. Its reactivity is characteristic of an ordinary polyene, i.e. it undergoes addition reactions. Benzene, by contrast, characteristically undergoes substitution reactions, not additions. History 1,3,5,7-Cyclooctatetraene was initially synthesized by Richard Willstätter in Munich in 1905 using pseudopelletierine as the starting material and the Hofmann elimination as the key transformation: : Willstätter noted that the compound did not exhibit the expected aromaticity. Between 1939 and 1943, chemists throughout the US ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pentalene
Pentalene is a polycyclic hydrocarbon composed of two fused cyclopentadiene rings. It has chemical formula . It is antiaromatic, because it has 4''n'' π electrons where ''n'' is any integer. For this reason it dimerizes even at temperatures as low as −100 °C. The derivative 1,3,5-tri-''tert''-butylpentalene was synthesized in 1973. Because of the ''tert''-butyl substituents this compound is thermally stable. Pentalenes can also be stabilized by benzannulation for example in the compounds benzopentalene and dibenzopentalene. Dilithium pentalenide was isolated in 1962, long before pentalene itself in 1997. It is prepared from reaction of dihydropentalene (pyrolysis of an isomer of dicyclopentadiene) with ''n''-butyllithium in solution and forms a stable salt. In accordance with its structure proton NMR shows 2 signals in a 2 to 1 ratio. The addition of two electrons removes the antiaromaticity; it becomes a planar 10π-electron aromatic species and is thus a bicyclic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biphenylene
Biphenylene is an organic compound with the formula (C6H4)2. It is a pale, yellowish solid with a hay-like odor. Despite its unusual structure, it behaves like a traditional polycyclic aromatic hydrocarbon. Bonding Biphenylene is a polycyclic hydrocarbon, composed of two benzene rings joined by two bridging bonds (as opposed to a normal ring fusion), thus forming a 6-4-6 arene system. The resulting planar structure was one of the first π-electronic hydrocarbon systems discovered to show evidence of antiaromaticity. The spectral and chemical properties show the influence of the central nring, leading to considerable interest in the system in terms of its degree of lessened aromaticity. Questions of bond alternation and ring currents have been investigated repeatedly. Both X-ray diffraction and electron diffraction studies show a considerable alternation of bond lengths, with the bridging bonds between the benzenoid rings having the unusually great length of 1.524 Å. The s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclooctatetraene
1,3,5,7-Cyclooctatetraene (COT) is an unsaturated derivative of cyclooctane, with the formula C8H8. It is also known as nnulene. This polyunsaturated hydrocarbon is a colorless to light yellow flammable liquid at room temperature. Because of its stoichiometric relationship to benzene, COT has been the subject of much research and some controversy. Unlike benzene, C6H6, cyclooctatetraene, C8H8, is not aromatic, although its dianion, ( cyclooctatetraenide), is. Its reactivity is characteristic of an ordinary polyene, i.e. it undergoes addition reactions. Benzene, by contrast, characteristically undergoes substitution reactions, not additions. History 1,3,5,7-Cyclooctatetraene was initially synthesized by Richard Willstätter in Munich in 1905 using pseudopelletierine as the starting material and the Hofmann elimination as the key transformation: : Willstätter noted that the compound did not exhibit the expected aromaticity. Between 1939 and 1943, chemists throug ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pentalene
Pentalene is a polycyclic hydrocarbon composed of two fused cyclopentadiene rings. It has chemical formula . It is antiaromatic, because it has 4''n'' π electrons where ''n'' is any integer. For this reason it dimerizes even at temperatures as low as −100 °C. The derivative 1,3,5-tri-''tert''-butylpentalene was synthesized in 1973. Because of the ''tert''-butyl substituents this compound is thermally stable. Pentalenes can also be stabilized by benzannulation for example in the compounds benzopentalene and dibenzopentalene. Dilithium pentalenide was isolated in 1962, long before pentalene itself in 1997. It is prepared from reaction of dihydropentalene (pyrolysis of an isomer of dicyclopentadiene) with ''n''-butyllithium in solution and forms a stable salt. In accordance with its structure proton NMR shows 2 signals in a 2 to 1 ratio. The addition of two electrons removes the antiaromaticity; it becomes a planar 10π-electron aromatic species and is thus a bicyclic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Baird's Rule
In organic chemistry, Baird's rule estimates whether the lowest triplet state of planar, cyclic structures will have aromatic properties or not. The quantum mechanical basis for its formulation was first worked out by physical chemist N. Colin Baird at the University of Western Ontario in 1972. The lowest triplet state of an annulene is, according to Baird's rule, aromatic when it has 4''n'' π-electrons and antiaromatic when the π-electron count is 4''n'' + 2, where ''n'' is any positive integer. This trend is opposite to that predicted by Hückel's rule for the ground state, which is usually the lowest singlet state (S0). Baird's rule has thus become known as the photochemical analogue of Hückel's rule. Through various theoretical investigations, this rule has also been found to extend to the lowest lying singlet excited state (S1) of small annulenes. See also * Möbius–Hückel concept * Möbius aromaticity In organic chemistry, Möbius aromaticity is a special typ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
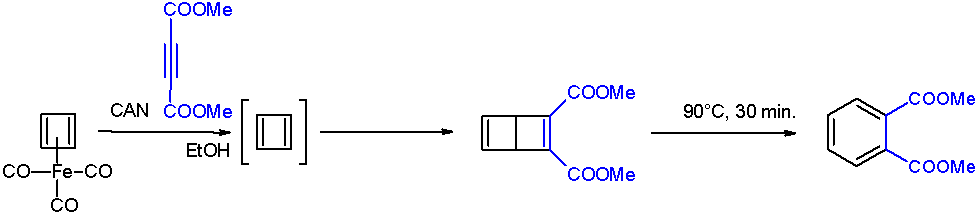
Cyclobutadiene
Cyclobutadiene is an organic compound with the formula . It is very reactive owing to its tendency to dimerize. Although the parent compound has not been isolated, some substituted derivatives are robust and a single molecule of cyclobutadiene is quite stable. Since the compound degrades by a bimolecular process, the species can be observed by matrix isolation techniques at temperatures below 35 K. It is thought to adopt a rectangular structure. Structure and reactivity The compound is the prototypical antiaromatic hydrocarbon with 4 π-electrons. It is the smallest 'n'' annulene ( annulene). Its rectangular structure is the result of the Jahn–Teller effect, which distorts the molecule and lowers its symmetry, converting the triplet to a singlet ground state. The electronic states of cyclobutadiene have been explored with a variety of computational methods. The rectangular structure is consistent with the existence of two different 1,2-dideutero-1,3-cyclobutadiene valence ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclododecahexaene
Cyclododecahexaene or 2nnulene () is a member of the series of annulenes with some interest in organic chemistry with regard to the study of aromaticity. Cyclododecahexaene is non-aromatic due to the lack of planarity of the structure. On the other hand the dianion with 14 electrons is a Hückel aromat and more stable. According to in silico experiments the tri-trans isomer is expected to be the most stable, followed by the 1,7-ditrans and the all cis-isomers (+1 kcal/mol) and by the 1,5-ditrans isomer (+5 kcal/mol). The first 2nnulene with sym-tri-trans configuration was synthesized in 1970 from a tricyclic precursor by photolysis at low temperatures. On heating the compound rearranges to a bicyclic .4.0isomer. Reducing the compound at low temperatures allowed analysis of the dianion by proton NMR with the inner protons resonating at -4.5 ppm relative to TMS, evidence of an aromatic diamagnetic ring current. : In one study the 1,7-ditrans isomer is generated at low tem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.png)