|
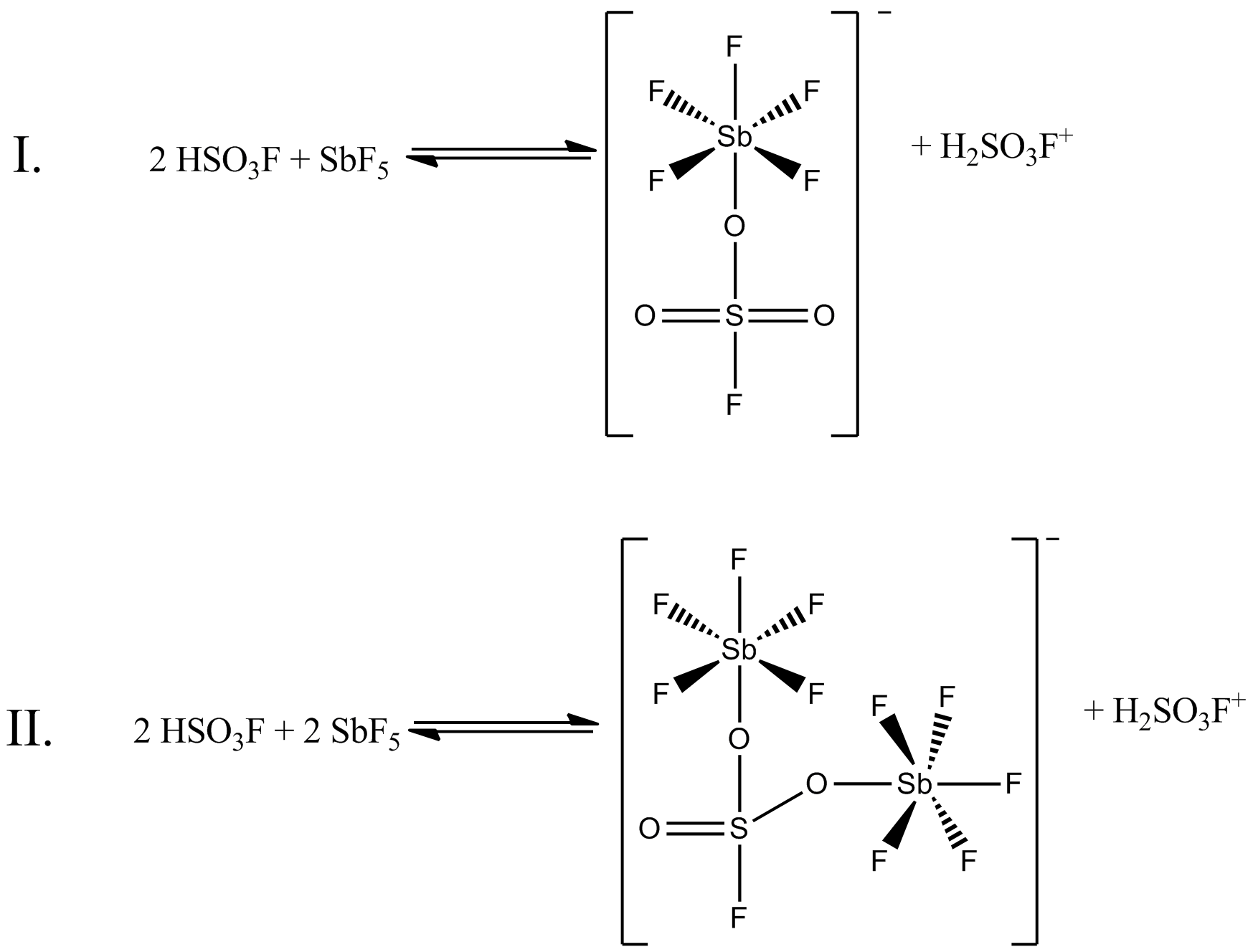
Magic Acid
Magic acid () is a superacid consisting of a mixture, most commonly in a 1:1 molar ratio, of fluorosulfuric acid () and antimony pentafluoride (). This conjugate Brønsted acid, Brønsted–Lewis acid, Lewis superacid system was developed in the 1960s by Ronald Gillespie and his team at McMaster University, and has been used by George Olah to stabilise carbocations and hypercoordination, hypercoordinated carbonium ions in liquid media. Magic acid and other superacids are also used to catalyze isomerization of saturated hydrocarbons, and have been shown to protonation, protonate even weak bases, including methane, xenon, halogens, and molecular hydrogen. History The term "superacid" was first used in 1927 when James Bryant Conant found that perchloric acid could protonate ketones and aldehydes to form salts in nonaqueous solution. The term itself was coined by R. J. Gillespie, after Conant combined sulfuric acid with fluorosulfuric acid, and found the solution to be several million ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Superacid
In chemistry, a superacid (according to the original definition) is an acid with an acidity greater than that of 100% pure sulfuric acid (), which has a Hammett acidity function (''H''0) of −12. According to the modern definition, a superacid is a medium in which the chemical potential of the proton is higher than in pure sulfuric acid. Commercially available superacids include trifluoromethanesulfonic acid (), also known as triflic acid, and fluorosulfuric acid (), both of which are about a thousand times stronger (i.e. have more negative ''H''0 values) than sulfuric acid. Most strong superacids are prepared by the combination of a strong Lewis acid and a strong Brønsted acid. A strong superacid of this kind is fluoroantimonic acid. Another group of superacids, the carborane acid group, contains some of the strongest known acids. Finally, when treated with anhydrous acid, zeolites (microporous aluminosilicate minerals) will contain superacidic sites within their pores. These m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
James Bryant Conant
James Bryant Conant (March 26, 1893 – February 11, 1978) was an American chemist, a transformative President of Harvard University, and the first U.S. Ambassador to West Germany. Conant obtained a Ph.D. in chemistry from Harvard in 1916. During World War I, he served in the U.S. Army, where he worked on the development of poison gases, especially lewisite. He became an assistant professor of chemistry at Harvard University in 1919 and the Sheldon Emery Professor of Organic Chemistry in 1929. He researched the physical structures of natural products, particularly chlorophyll, and he was one of the first to explore the sometimes complex relationship between chemical equilibrium and the reaction rate of chemical processes. He studied the biochemistry of oxyhemoglobin providing insight into the disease methemoglobinemia, helped to explain the structure of chlorophyll, and contributed important insights that underlie modern theories of acid-base chemistry. In 1933, Cona ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IR Spectroscopy
Infrared spectroscopy (IR spectroscopy or vibrational spectroscopy) is the measurement of the interaction of infrared radiation with matter by absorption, emission, or reflection. It is used to study and identify chemical substances or functional groups in solid, liquid, or gaseous forms. It can be used to characterize new materials or identify and verify known and unknown samples. The method or technique of infrared spectroscopy is conducted with an instrument called an infrared spectrometer (or spectrophotometer) which produces an infrared spectrum. An IR spectrum can be visualized in a graph of infrared light absorbance (or transmittance) on the vertical axis vs. frequency, wavenumber or wavelength on the horizontal axis. Typical units of wavenumber used in IR spectra are reciprocal centimeters, with the symbol cm−1. Units of IR wavelength are commonly given in micrometers (formerly called "microns"), symbol μm, which are related to the wavenumber in a reciprocal way. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nonclassical Ion
In chemistry, a nonclassical ion usually refers to carbonium ions, a family of organic cations. They are characterized by delocalized three-center, two-electron bonds. The more stable members are often bi- or polycyclic. Examples Historically, nonclassical ions were invoked to explain unusually fast solvolyses of steroidal, norbornyl, and cyclopropyl halides. Explanations for these rates was once controversial. The 2-norbornyl cation is one of the best characterized carbonium ions: : In fact, it has emerged as the prototype for non-classical ions. As indicated first by low-temperature NMR spectroscopy and confirmed by X-ray crystallography, it has a symmetric structure with an RCH2+ group bonded to an alkene group, stabilized by a bicyclic structure. Solvolyses of cyclopropylcarbinyl, cyclobutyl, and homoallyl esters are also characterized by very large rates, and have been shown to occur via a common nonclassical ion structure in the form of a bicyclobutonium ion. Siehl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopentyl Cation
Cyclopentane (also called C pentane) is a highly flammable alicyclic hydrocarbon with chemical formula C5H10 and CAS number 287-92-3, consisting of a ring of five carbon atoms each bonded with two hydrogen atoms above and below the plane. It is a colorless liquid with a petrol-like odor. Its freezing point is −94 °C and its boiling point is 49 °C. Cyclopentane is in the class of cycloalkanes, being alkanes that have one or more carbon rings. It is formed by cracking cyclohexane in the presence of alumina at a high temperature and pressure. It was first prepared in 1893 by the German chemist Johannes Wislicenus. Production, occurrence and use Cycloalkanes are formed by catalytic reforming. For example, when passed over a hot platinum surface, 2-methylbutane converts into cyclopentane. Cyclopentane is principally used as a blowing agent in the manufacture of polyurethane insulating foam, replacing ozone-depleting agents such as CFC-11 and HCFC-141b. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclohexane
Cyclohexane is a cycloalkane with the molecular formula . Cyclohexane is non-polar. Cyclohexane is a colourless, flammable liquid with a distinctive detergent-like odor, reminiscent of cleaning products (in which it is sometimes used). Cyclohexane is mainly used for the industrial production of adipic acid and caprolactam, which are Precursor (chemistry), precursors to nylon. Cyclohexyl () is the alkyl substituent of cyclohexane and is abbreviated Cy. Production Cyclohexane is one of components of naphtha, from which it can be extracted by advanced distillation methods. Distillation is usually combined with isomerization of methylcyclopentane, a similar component extracted from naphtha by similar methods. Together, these processes cover only a minority (15-20%) of the modern industrial demand, and are complemented by synthesis. Modern industrial synthesis On an industrial scale, cyclohexane is produced by hydrogenation of benzene in the presence of a Raney nickel catalyst. Prod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lewis Dot Structures
Lewis structuresalso called Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDs)are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule. Introduced by Gilbert N. Lewis in his 1916 article ''The Atom and the Molecule'', a Lewis structure can be drawn for any covalently bonded molecule, as well as coordination compounds. Lewis structures extend the concept of the electron dot diagram by adding lines between atoms to represent shared pairs in a chemical bond. Lewis structures show each atom and its position in the structure of the molecule using its chemical symbol. Lines are drawn between atoms that are bonded to one another (pairs of dots can be used instead of lines). Excess electrons that form lone pairs are represented as pairs of dots, and are placed next to the atoms. Although main group elements of the second period and beyond usually react b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluoroantimonic Acid
Fluoroantimonic acid is a mixture of hydrogen fluoride and antimony pentafluoride, containing various cations and anions (the simplest being and ). This mixture is a superacid stronger than pure sulfuric acid, by many orders of magnitude, according to its Hammett acidity function. It even protonates some hydrocarbons to afford pentacoordinate carbocations ( carbonium ions). Like its precursor hydrogen fluoride, it attacks glass, but can be stored in containers lined with PTFE (Teflon) or PFA. Chemical composition Fluoroantimonic acid is formed by combining hydrogen fluoride and antimony pentafluoride: :SbF5 + 2 HF + H2F+ The speciation (i.e., the inventory of components) of fluoroantimonic acid is complex. Spectroscopic measurements show that fluoroantimonic acid consists of a mixture of HF-solvated protons, – (such as ). Thus, the formula "" is a convenient but oversimplified approximation of the true composition. Nevertheless, the extreme acidity of this mixt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hammett Acidity Function
The Hammett acidity function (''H''0) is a measure of acidity that is used for very concentrated solutions of strong acids, including superacids. It was proposed by the physical organic chemist Louis Plack Hammett and is the best-known acidity function used to extend the measure of Brønsted–Lowry acidity beyond the dilute aqueous solutions for which the pH scale is useful. In highly concentrated solutions, simple approximations such as the Henderson–Hasselbalch equation are no longer valid due to the variations of the activity coefficients. The Hammett acidity function is used in fields such as physical organic chemistry for the study of acid-catalyzed reactions, because some of these reactions use acids in very high concentrations, or even neat (pure).Gerrylynn K. Roberts, Colin Archibald Russell. ''Chemical History: Reviews of the Recent Literature''. Royal Society of Chemistry, 2005. . Definition The Hammett acidity function, ''H''0, can replace the pH in concentrat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Formal Charge
In chemistry, a formal charge (F.C. or ), in the covalent view of chemical bonding, is the hypothetical charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between atoms, regardless of relative electronegativity. In simple terms, formal charge is the difference between the number of valence electrons of an atom in a neutral free state and the number assigned to that atom in a Lewis structure. When determining the best Lewis structure (or predominant resonance structure) for a molecule, the structure is chosen such that the formal charge on each of the atoms is as close to zero as possible. The formal charge of any atom in a molecule can be calculated by the following equation: q^ = V - L - \frac where is the number of valence electrons of the neutral atom in isolation (in its ground state); is the number of non-bonding valence electrons assigned to this atom in the Lewis structure of the molecule; and is the total num ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magic Acid Structure
Magic or magick most commonly refers to: * Magic (supernatural), beliefs and actions employed to influence supernatural beings and forces ** ''Magick'' (with ''-ck'') can specifically refer to ceremonial magic * Magic (illusion), also known as stage magic, the art of appearing to perform supernatural feats * Magical thinking, the belief that unrelated events are causally connected, particularly as a result of supernatural effects Magic or magick may also refer to: Art and entertainment Film and television * ''Magic'' (1917 film), a silent Hungarian drama * ''Magic'' (1978 film), an American horror film * ''Magic'', a 1983 Taiwanese film starring Wen Chao-yu * Magic (TV channel), a British music television station Literature * Magic in fiction, the genre of fiction that uses supernatural elements as a theme * '' Magic: A Fantastic Comedy'', a 1913 play by G. K. Chesterton * ''Magic'' (short story collection), a 1996 short story collection by Isaac Asimov * ''Magic'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paraffin Wax
Paraffin wax (or petroleum wax) is a soft colorless solid derived from petroleum, coal, or oil shale that consists of a mixture of hydrocarbon molecules containing between 20 and 40 carbon atoms. It is solid at room temperature and melting point, begins to melt above approximately , and its boiling point is above . Common applications for paraffin wax include lubrication, electrical insulation, and candles; dyed paraffin wax can be made into crayons. Un-dyed, unscented paraffin candles are odorless and bluish-white. Paraffin wax was first created by Carl Reichenbach#Scientific contributions, Carl Reichenbach in Germany in 1830 and marked a major advancement in candlemaking technology, as it burned more cleanly and reliably than tallow candles and was cheaper to produce. In chemistry, ''paraffin'' is used synonymously with ''alkane'', indicating hydrocarbons with the general formula C''n''H2''n''+2. The name is derived from Latin ''parum'' ("very little") + ''affinis'', meaning ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |