Carbon dioxide is a
chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
with the
chemical formula
A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as pare ...
. It is made up of
molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
s that each have one
carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
atom
covalently double bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betw ...
ed to two
oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
atoms. It is found in a gas state at room temperature and at normally-encountered concentrations it is odorless. As the source of carbon in the
carbon cycle
The carbon cycle is a part of the biogeochemical cycle where carbon is exchanged among the biosphere, pedosphere, geosphere, hydrosphere, and atmosphere of Earth. Other major biogeochemical cycles include the nitrogen cycle and the water cycl ...
, atmospheric is the primary carbon source for life on Earth. In the air, carbon dioxide is transparent to visible light but absorbs
infrared radiation
Infrared (IR; sometimes called infrared light) is electromagnetic radiation (EMR) with wavelengths longer than that of visible light but shorter than microwaves. The infrared spectral band begins with the waves that are just longer than those ...
, acting as a
greenhouse gas
Greenhouse gases (GHGs) are the gases in the atmosphere that raise the surface temperature of planets such as the Earth. Unlike other gases, greenhouse gases absorb the radiations that a planet emits, resulting in the greenhouse effect. T ...
. Carbon dioxide is soluble in water and is found in
groundwater
Groundwater is the water present beneath Earth's surface in rock and Pore space in soil, soil pore spaces and in the fractures of stratum, rock formations. About 30 percent of all readily available fresh water in the world is groundwater. A unit ...
,
lake
A lake is often a naturally occurring, relatively large and fixed body of water on or near the Earth's surface. It is localized in a basin or interconnected basins surrounded by dry land. Lakes lie completely on land and are separate from ...
s,
ice caps, and
seawater
Seawater, or sea water, is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5% (35 g/L, 35 ppt, 600 mM). This means that every kilogram (roughly one liter by volume) of seawater has approximat ...
.
It is a
trace gas in Earth's atmosphere at 421
parts per million
In science and engineering, the parts-per notation is a set of pseudo-units to describe the small values of miscellaneous dimensionless quantity, dimensionless quantities, e.g. mole fraction or mass fraction (chemistry), mass fraction.
Since t ...
(ppm), or about 0.042% (as of May 2022) having risen from pre-industrial levels of 280 ppm or about 0.028%.
Burning
fossil fuels is the main cause of these increased concentrations, which are the primary cause of
climate change
Present-day climate change includes both global warming—the ongoing increase in Global surface temperature, global average temperature—and its wider effects on Earth's climate system. Climate variability and change, Climate change in ...
.
[IPCC (2022]
Summary for policy makers
i
Climate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change
Cambridge University Press, Cambridge, United Kingdom and New York, NY, US
Its
concentration
In chemistry, concentration is the abundance of a constituent divided by the total volume of a mixture. Several types of mathematical description can be distinguished: '' mass concentration'', '' molar concentration'', '' number concentration'', ...
in Earth's pre-industrial atmosphere since late in the
Precambrian
The Precambrian ( ; or pre-Cambrian, sometimes abbreviated pC, or Cryptozoic) is the earliest part of Earth's history, set before the current Phanerozoic Eon. The Precambrian is so named because it preceded the Cambrian, the first period of t ...
was regulated by organisms and geological features.
Plant
Plants are the eukaryotes that form the Kingdom (biology), kingdom Plantae; they are predominantly Photosynthesis, photosynthetic. This means that they obtain their energy from sunlight, using chloroplasts derived from endosymbiosis with c ...
s,
algae
Algae ( , ; : alga ) is an informal term for any organisms of a large and diverse group of photosynthesis, photosynthetic organisms that are not plants, and includes species from multiple distinct clades. Such organisms range from unicellular ...
and
cyanobacteria
Cyanobacteria ( ) are a group of autotrophic gram-negative bacteria that can obtain biological energy via oxygenic photosynthesis. The name "cyanobacteria" () refers to their bluish green (cyan) color, which forms the basis of cyanobacteri ...
use
energy
Energy () is the physical quantity, quantitative physical property, property that is transferred to a physical body, body or to a physical system, recognizable in the performance of Work (thermodynamics), work and in the form of heat and l ...
from
sunlight
Sunlight is the portion of the electromagnetic radiation which is emitted by the Sun (i.e. solar radiation) and received by the Earth, in particular the visible spectrum, visible light perceptible to the human eye as well as invisible infrare ...
to synthesize
carbohydrate
A carbohydrate () is a biomolecule composed of carbon (C), hydrogen (H), and oxygen (O) atoms. The typical hydrogen-to-oxygen atomic ratio is 2:1, analogous to that of water, and is represented by the empirical formula (where ''m'' and ''n'' ...
s from carbon dioxide and water in a process called
photosynthesis
Photosynthesis ( ) is a system of biological processes by which photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical energy necessary to fuel their metabo ...
, which produces oxygen as a waste product. In turn, oxygen is consumed and is released as waste by all
aerobic organism
An aerobic organism or aerobe is an organism that can survive and grow in an oxygenated environment. The ability to exhibit aerobic respiration may yield benefits to the aerobic organism, as aerobic respiration yields more energy than anaerobic ...
s when they metabolize
organic compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-co ...
s to produce energy by
respiration. is released from organic materials when they
decay or combust, such as in forest fires. When carbon dioxide dissolves in water, it forms
carbonate
A carbonate is a salt of carbonic acid, (), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word "carbonate" may also refer to a carbonate ester, an organic compound containing the carbonate group ...
and mainly
bicarbonate
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula .
Bicarbonate serves a crucial bioche ...
(), which causes
ocean acidification as
atmospheric levels increase.
Carbon dioxide is 53% more dense than dry air, but is long lived and thoroughly mixes in the atmosphere. About half of excess emissions to the atmosphere are absorbed by
land
Land, also known as dry land, ground, or earth, is the solid terrestrial surface of Earth not submerged by the ocean or another body of water. It makes up 29.2% of Earth's surface and includes all continents and islands. Earth's land sur ...
and ocean
carbon sink
A carbon sink is a natural or artificial carbon sequestration process that "removes a greenhouse gas, an aerosol or a precursor of a greenhouse gas from the atmosphere". These sinks form an important part of the natural carbon cycle. An overar ...
s. These sinks can become saturated and are volatile, as decay and
wildfire
A wildfire, forest fire, or a bushfire is an unplanned and uncontrolled fire in an area of Combustibility and flammability, combustible vegetation. Depending on the type of vegetation present, a wildfire may be more specifically identified as a ...
s result in the being released back into the atmosphere. , or the carbon it holds, is eventually
sequestered (stored for the long term) in rocks and organic deposits like
coal
Coal is a combustible black or brownish-black sedimentary rock, formed as rock strata called coal seams. Coal is mostly carbon with variable amounts of other Chemical element, elements, chiefly hydrogen, sulfur, oxygen, and nitrogen.
Coal i ...
,
petroleum
Petroleum, also known as crude oil or simply oil, is a naturally occurring, yellowish-black liquid chemical mixture found in geological formations, consisting mainly of hydrocarbons. The term ''petroleum'' refers both to naturally occurring un ...
and
natural gas
Natural gas (also fossil gas, methane gas, and gas) is a naturally occurring compound of gaseous hydrocarbons, primarily methane (95%), small amounts of higher alkanes, and traces of carbon dioxide and nitrogen, hydrogen sulfide and helium ...
.
Nearly all produced by humans goes into the atmosphere. Less than 1% of produced annually is put to commercial use, mostly in the fertilizer industry and in the oil and gas industry for
enhanced oil recovery. Other commercial applications include food and beverage production, metal fabrication, cooling, fire suppression and stimulating plant growth in greenhouses.
Chemical and physical properties
Structure, bonding and molecular vibrations
The
symmetry
Symmetry () in everyday life refers to a sense of harmonious and beautiful proportion and balance. In mathematics, the term has a more precise definition and is usually used to refer to an object that is Invariant (mathematics), invariant und ...
of a carbon dioxide molecule is linear and
centrosymmetric at its equilibrium geometry. The
length
Length is a measure of distance. In the International System of Quantities, length is a quantity with Dimension (physical quantity), dimension distance. In most systems of measurement a Base unit (measurement), base unit for length is chosen, ...
of the
carbon–oxygen bond in carbon dioxide is 116.3
pm, noticeably shorter than the roughly 140 pm length of a typical single C–O bond, and shorter than most other C–O multiply bonded
functional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
s such as
carbonyls.
[ Since it is centrosymmetric, the molecule has no ]electric dipole moment
The electric dipole moment is a measure of the separation of positive and negative electrical charges within a system: that is, a measure of the system's overall Chemical polarity, polarity. The International System of Units, SI unit for electric ...
.
 As a linear triatomic molecule, has four vibrational modes as shown in the diagram. In the symmetric and the antisymmetric stretching modes, the atoms move along the axis of the molecule. There are two bending modes, which are degenerate, meaning that they have the same frequency and same energy, because of the symmetry of the molecule. When a molecule touches a surface or touches another molecule, the two bending modes can differ in frequency because the interaction is different for the two modes. Some of the vibrational modes are observed in the infrared (IR) spectrum: the antisymmetric stretching mode at
As a linear triatomic molecule, has four vibrational modes as shown in the diagram. In the symmetric and the antisymmetric stretching modes, the atoms move along the axis of the molecule. There are two bending modes, which are degenerate, meaning that they have the same frequency and same energy, because of the symmetry of the molecule. When a molecule touches a surface or touches another molecule, the two bending modes can differ in frequency because the interaction is different for the two modes. Some of the vibrational modes are observed in the infrared (IR) spectrum: the antisymmetric stretching mode at wavenumber
In the physical sciences, the wavenumber (or wave number), also known as repetency, is the spatial frequency of a wave. Ordinary wavenumber is defined as the number of wave cycles divided by length; it is a physical quantity with dimension of ...
2349 cm−1 (wavelength 4.25 μm) and the degenerate pair of bending modes at 667 cm−1 (wavelength 15.0 μm). The symmetric stretching mode does not create an electric dipole so is not observed in IR spectroscopy, but it is detected in Raman spectroscopy
Raman spectroscopy () (named after physicist C. V. Raman) is a Spectroscopy, spectroscopic technique typically used to determine vibrational modes of molecules, although rotational and other low-frequency modes of systems may also be observed. Ra ...
at 1388 cm−1 (wavelength 7.20 μm), with a Fermi resonance doublet at 1285 cm−1.
In the gas phase, carbon dioxide molecules undergo significant vibrational motions and do not keep a fixed structure. However, in a Coulomb explosion imaging experiment, an instantaneous image of the molecular structure can be deduced. Such an experiment has been performed for carbon dioxide. The result of this experiment, and the conclusion of theoretical calculations[ This is so for all molecules except ]diatomic molecule
Diatomic molecules () are molecules composed of only two atoms, of the same or different chemical elements. If a diatomic molecule consists of two atoms of the same element, such as hydrogen () or oxygen (), then it is said to be homonuclear mol ...
s.
In aqueous solution
Carbon dioxide is soluble in water, in which it reversibly forms (carbonic acid), which is a weak acid, because its ionization in water is incomplete.
:
The hydration equilibrium constant of carbonic acid is, at 25 °C:
:
Hence, the majority of the carbon dioxide is not converted into carbonic acid, but remains as molecules, not affecting the pH.
The relative concentrations of , , and the deprotonated forms (bicarbonate
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula .
Bicarbonate serves a crucial bioche ...
) and (carbonate
A carbonate is a salt of carbonic acid, (), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word "carbonate" may also refer to a carbonate ester, an organic compound containing the carbonate group ...
) depend on the pH. As shown in a Bjerrum plot, in neutral or slightly alkaline water (pH > 6.5), the bicarbonate form predominates (>50%) becoming the most prevalent (>95%) at the pH of seawater. In very alkaline water (pH > 10.4), the predominant (>50%) form is carbonate. The oceans, being mildly alkaline with typical pH = 8.2–8.5, contain about 120 mg of bicarbonate per liter.
Being diprotic, carbonic acid has two acid dissociation constants, the first one for the dissociation into the bicarbonate (also called hydrogen carbonate) ion ():
:
:''K''a1 = 2.5 × 10−4 mol/L; p''K''a1 = 3.6 at 25 °C.carbonate
A carbonate is a salt of carbonic acid, (), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word "carbonate" may also refer to a carbonate ester, an organic compound containing the carbonate group ...
ion ():
:
:''K''a2 = 4.69 × 10−11 mol/L; p''K''a2 = 10.329
In organisms, carbonic acid production is catalysed by the enzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different mol ...
known as carbonic anhydrase.
In addition to altering its acidity, the presence of carbon dioxide in water also affects its electrical properties.  When carbon dioxide dissolves in desalinated water, the electrical conductivity increases significantly from below 1 μS/cm to nearly 30 μS/cm. When heated, the water begins to gradually lose the conductivity induced by the presence of , especially noticeable as temperatures exceed 30 °C.
The temperature dependence of the electrical conductivity of fully deionized water without saturation is comparably low in relation to these data.
When carbon dioxide dissolves in desalinated water, the electrical conductivity increases significantly from below 1 μS/cm to nearly 30 μS/cm. When heated, the water begins to gradually lose the conductivity induced by the presence of , especially noticeable as temperatures exceed 30 °C.
The temperature dependence of the electrical conductivity of fully deionized water without saturation is comparably low in relation to these data.
Chemical reactions
is a potent electrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively Electric charge, charged, have an ...
having an electrophilic reactivity that is comparable to benzaldehyde
Benzaldehyde (C6H5CHO) is an organic compound consisting of a benzene ring with a formyl substituent. It is among the simplest aromatic aldehydes and one of the most industrially useful.
It is a colorless liquid with a characteristic almond-li ...
or strongly electrophilic α,β-unsaturated carbonyl compounds. However, unlike electrophiles of similar reactivity, the reactions of nucleophiles with are thermodynamically less favored and are often found to be highly reversible. The reversible reaction of carbon dioxide with amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
s to make carbamates is used in scrubbers and has been suggested as a possible starting point for carbon capture and storage by amine gas treating.
Only very strong nucleophiles, like the carbanions provided by Grignard reagents and organolithium compound
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom ...
s react with to give carboxylates:
:
:where M = Li or Mg Br and R = alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl group is derived from a cy ...
or aryl.
In metal carbon dioxide complexes, serves as a ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
, which can facilitate the conversion of to other chemicals.
The reduction of to CO is ordinarily a difficult and slow reaction:
:
The redox potential for this reaction near pH 7 is about −0.53 V ''versus'' the standard hydrogen electrode. The nickel-containing enzyme carbon monoxide dehydrogenase catalyses this process.
Photoautotrophs (i.e. plant
Plants are the eukaryotes that form the Kingdom (biology), kingdom Plantae; they are predominantly Photosynthesis, photosynthetic. This means that they obtain their energy from sunlight, using chloroplasts derived from endosymbiosis with c ...
s and cyanobacteria
Cyanobacteria ( ) are a group of autotrophic gram-negative bacteria that can obtain biological energy via oxygenic photosynthesis. The name "cyanobacteria" () refers to their bluish green (cyan) color, which forms the basis of cyanobacteri ...
) use the energy contained in sunlight to photosynthesize simple sugar
Sugar is the generic name for sweet-tasting, soluble carbohydrates, many of which are used in food. Simple sugars, also called monosaccharides, include glucose
Glucose is a sugar with the Chemical formula#Molecular formula, molecul ...
s from absorbed from the air and water:
:
Physical properties
 Carbon dioxide is colorless. At low concentrations, the gas is odorless; however, at sufficiently high concentrations, it has a sharp, acidic odor.
Carbon dioxide is colorless. At low concentrations, the gas is odorless; however, at sufficiently high concentrations, it has a sharp, acidic odor.dry ice
Dry ice is the solid form of carbon dioxide. It is commonly used for temporary refrigeration as CO2 does not have a liquid state at normal atmospheric pressure and Sublimation (phase transition), sublimes directly from the solid state to the gas ...
.
 Liquid carbon dioxide forms only at
Liquid carbon dioxide forms only at pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country and eve ...
s above 0.51795(10) MPatriple point
In thermodynamics, the triple point of a substance is the temperature and pressure at which the three Phase (matter), phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium.. It is that temperature and pressure at ...
of carbon dioxide is 216.592(3) Kamorphous
In condensed matter physics and materials science, an amorphous solid (or non-crystalline solid) is a solid that lacks the long-range order that is a characteristic of a crystal. The terms "glass" and "glassy solid" are sometimes used synonymousl ...
glass-like solid. This form of glass, called '' carbonia'', is produced by supercooling heated at extreme pressures (40–48 GPa, or about 400,000 atmospheres) in a diamond anvil. This discovery confirmed the theory that carbon dioxide could exist in a glass state similar to other members of its elemental family, like silicon dioxide
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundan ...
(silica glass) and germanium dioxide. Unlike silica and germania glasses, however, carbonia glass is not stable at normal pressures and reverts to gas when pressure is released.
At temperatures and pressures above the critical point, carbon dioxide behaves as a supercritical fluid known as supercritical carbon dioxide.
Table of thermal and physical properties of saturated liquid carbon dioxide:[
]
Biological role
Carbon dioxide is an end product of cellular respiration
Cellular respiration is the process of oxidizing biological fuels using an inorganic electron acceptor, such as oxygen, to drive production of adenosine triphosphate (ATP), which stores chemical energy in a biologically accessible form. Cell ...
in organisms that obtain energy by breaking down sugars, fats and amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 a ...
s with oxygen as part of their metabolism
Metabolism (, from ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run cellular processes; the co ...
. This includes all plants, algae and animals and aerobic fungi and bacteria. In vertebrate
Vertebrates () are animals with a vertebral column (backbone or spine), and a cranium, or skull. The vertebral column surrounds and protects the spinal cord, while the cranium protects the brain.
The vertebrates make up the subphylum Vertebra ...
s, the carbon dioxide travels in the blood from the body's tissues to the skin (e.g., amphibian
Amphibians are ectothermic, anamniote, anamniotic, tetrapod, four-limbed vertebrate animals that constitute the class (biology), class Amphibia. In its broadest sense, it is a paraphyletic group encompassing all Tetrapod, tetrapods, but excl ...
s) or the gills (e.g., fish
A fish (: fish or fishes) is an aquatic animal, aquatic, Anamniotes, anamniotic, gill-bearing vertebrate animal with swimming fish fin, fins and craniate, a hard skull, but lacking limb (anatomy), limbs with digit (anatomy), digits. Fish can ...
), from where it dissolves in the water, or to the lungs from where it is exhaled. During active photosynthesis, plants can absorb more carbon dioxide from the atmosphere than they release in respiration.
Photosynthesis and carbon fixation

Carbon fixation
Biological carbon fixation, or сarbon assimilation, is the Biological process, process by which living organisms convert Total inorganic carbon, inorganic carbon (particularly carbon dioxide, ) to Organic compound, organic compounds. These o ...
is a biochemical process by which atmospheric carbon dioxide is incorporated by plants, algae and cyanobacteria into energy-rich organic molecules such as glucose
Glucose is a sugar with the Chemical formula#Molecular formula, molecular formula , which is often abbreviated as Glc. It is overall the most abundant monosaccharide, a subcategory of carbohydrates. It is mainly made by plants and most algae d ...
, thus creating their own food by photosynthesis. Photosynthesis uses carbon dioxide and water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known liv ...
to produce sugars from which other organic compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-co ...
s can be constructed, and oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
is produced as a by-product.
Ribulose-1,5-bisphosphate carboxylase oxygenase, commonly abbreviated to RuBisCO, is the enzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different mol ...
involved in the first major step of carbon fixation, the production of two molecules of 3-phosphoglycerate from and ribulose bisphosphate, as shown in the diagram at left.
RuBisCO is thought to be the single most abundant protein on Earth.
Phototrophs use the products of their photosynthesis as internal food sources and as raw material for the biosynthesis
Biosynthesis, i.e., chemical synthesis occurring in biological contexts, is a term most often referring to multi-step, enzyme-Catalysis, catalyzed processes where chemical substances absorbed as nutrients (or previously converted through biosynthe ...
of more complex organic molecules, such as polysaccharide
Polysaccharides (), or polycarbohydrates, are the most abundant carbohydrates found in food. They are long-chain polymeric carbohydrates composed of monosaccharide units bound together by glycosidic linkages. This carbohydrate can react with wat ...
s, nucleic acid
Nucleic acids are large biomolecules that are crucial in all cells and viruses. They are composed of nucleotides, which are the monomer components: a pentose, 5-carbon sugar, a phosphate group and a nitrogenous base. The two main classes of nuclei ...
s, and proteins. These are used for their own growth, and also as the basis of the food chain
A food chain is a linear network of links in a food web, often starting with an autotroph (such as grass or algae), also called a producer, and typically ending at an apex predator (such as grizzly bears or killer whales), detritivore (such as ...
s and webs that feed other organisms, including animals such as ourselves. Some important phototrophs, the coccolithophore
Coccolithophores, or coccolithophorids, are single-celled organisms which are part of the phytoplankton, the autotrophic (self-feeding) component of the plankton community. They form a group of about 200 species, and belong either to the kingdom ...
s synthesise hard calcium carbonate
Calcium carbonate is a chemical compound with the chemical formula . It is a common substance found in Rock (geology), rocks as the minerals calcite and aragonite, most notably in chalk and limestone, eggshells, gastropod shells, shellfish skel ...
scales. A globally significant species of coccolithophore is ''Emiliania huxleyi
''Gephyrocapsa huxleyi'', also called ''Emiliania huxleyi'', is the most abundant species of coccolithophore in modern oceans found in almost all ecosystems from the equator to sub-polar regions, and from nutrient rich upwelling zones to nutr ...
'' whose calcite
Calcite is a Carbonate minerals, carbonate mineral and the most stable Polymorphism (materials science), polymorph of calcium carbonate (CaCO3). It is a very common mineral, particularly as a component of limestone. Calcite defines hardness 3 on ...
scales have formed the basis of many sedimentary rock
Sedimentary rocks are types of rock (geology), rock formed by the cementation (geology), cementation of sediments—i.e. particles made of minerals (geological detritus) or organic matter (biological detritus)—that have been accumulated or de ...
s such as limestone
Limestone is a type of carbonate rock, carbonate sedimentary rock which is the main source of the material Lime (material), lime. It is composed mostly of the minerals calcite and aragonite, which are different Polymorphism (materials science) ...
, where what was previously atmospheric carbon can remain fixed for geological timescales.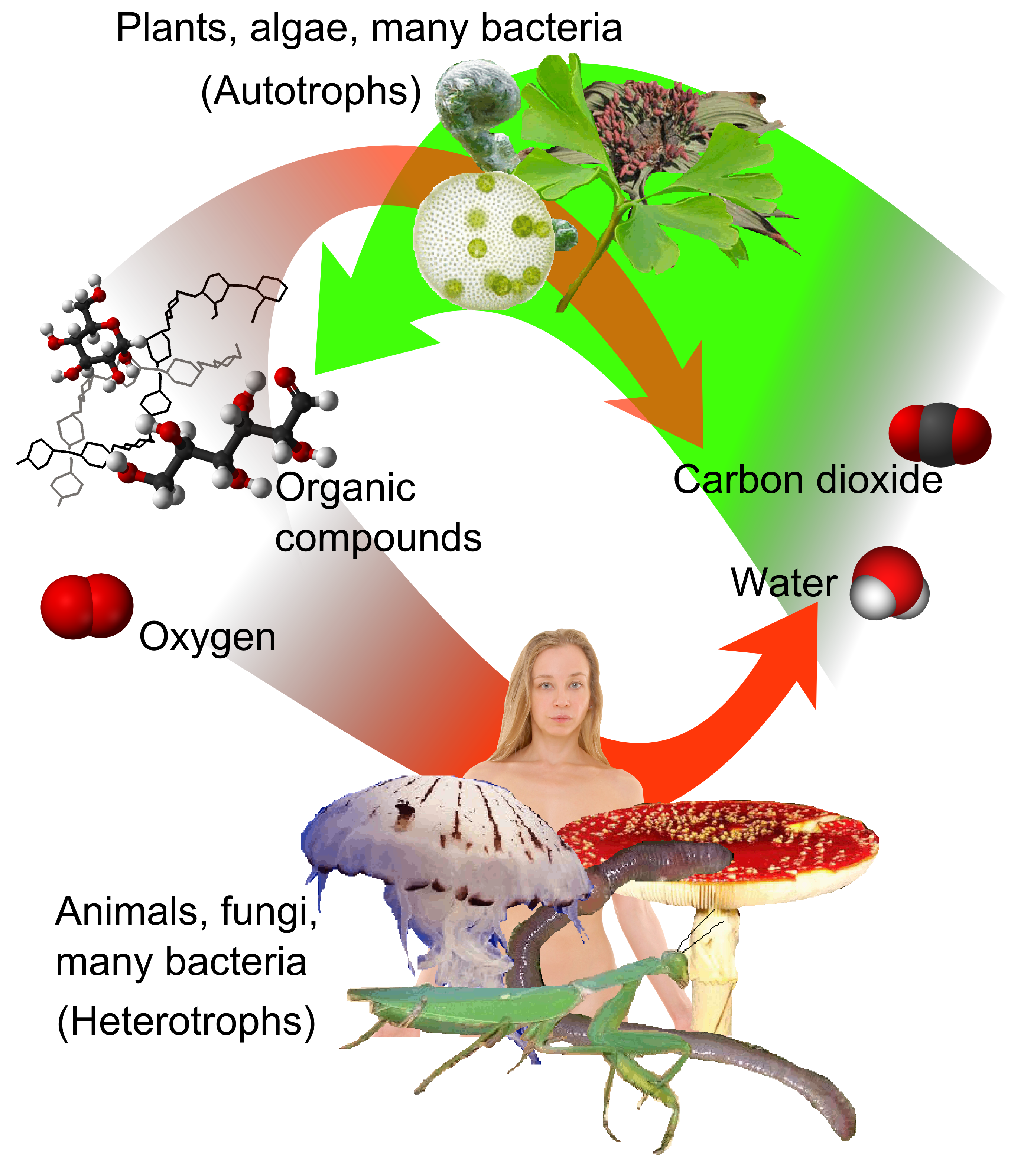 Plants can grow as much as 50% faster in concentrations of 1,000 ppm when compared with ambient conditions, though this assumes no change in climate and no limitation on other nutrients. Elevated levels cause increased growth reflected in the harvestable yield of crops, with wheat, rice and soybean all showing increases in yield of 12–14% under elevated in FACE experiments.
Increased atmospheric concentrations result in fewer stomata developing on plants which leads to reduced water usage and increased water-use efficiency. Studies using FACE have shown that enrichment leads to decreased concentrations of micronutrients in crop plants. This may have knock-on effects on other parts of
Plants can grow as much as 50% faster in concentrations of 1,000 ppm when compared with ambient conditions, though this assumes no change in climate and no limitation on other nutrients. Elevated levels cause increased growth reflected in the harvestable yield of crops, with wheat, rice and soybean all showing increases in yield of 12–14% under elevated in FACE experiments.
Increased atmospheric concentrations result in fewer stomata developing on plants which leads to reduced water usage and increased water-use efficiency. Studies using FACE have shown that enrichment leads to decreased concentrations of micronutrients in crop plants. This may have knock-on effects on other parts of ecosystem
An ecosystem (or ecological system) is a system formed by Organism, organisms in interaction with their Biophysical environment, environment. The Biotic material, biotic and abiotic components are linked together through nutrient cycles and en ...
s as herbivores will need to eat more food to gain the same amount of protein.
The concentration of secondary metabolites
In biochemistry, a metabolite is an intermediate or end product of metabolism.
The term is usually used for small molecules. Metabolites have various functions, including fuel, structure, signaling, stimulatory and inhibitory effects on enzymes, c ...
such as phenylpropanoid
The phenylpropanoids are a diverse family of organic compounds that are biosynthesized by plants from the amino acids phenylalanine and tyrosine in the shikimic acid pathway. Their name is derived from the six-carbon, aromatic phenyl group and ...
s and flavonoid
Flavonoids (or bioflavonoids; from the Latin word ''flavus'', meaning yellow, their color in nature) are a class of polyphenolic secondary metabolites found in plants, and thus commonly consumed in the diets of humans.
Chemically, flavonoids ...
s can also be altered in plants exposed to high concentrations of .
Plants also emit during respiration, and so the majority of plants and algae, which use C3 photosynthesis, are only net absorbers during the day. Though a growing forest will absorb many tons of each year, a mature forest will produce as much from respiration and decomposition of dead specimens (e.g., fallen branches) as is used in photosynthesis in growing plants. Contrary to the long-standing view that they are carbon neutral, mature forests can continue to accumulate carbon and remain valuable carbon sink
A carbon sink is a natural or artificial carbon sequestration process that "removes a greenhouse gas, an aerosol or a precursor of a greenhouse gas from the atmosphere". These sinks form an important part of the natural carbon cycle. An overar ...
s, helping to maintain the carbon balance of Earth's atmosphere. Additionally, and crucially to life on earth, photosynthesis by phytoplankton consumes dissolved in the upper ocean and thereby promotes the absorption of from the atmosphere.
Toxicity
 Carbon dioxide content in fresh air (averaged between sea-level and 10 kPa level, i.e., about altitude) varies between 0.036% (360 ppm) and 0.041% (412 ppm), depending on the location.
In humans, exposure to at concentrations greater than 5% causes the development of
Carbon dioxide content in fresh air (averaged between sea-level and 10 kPa level, i.e., about altitude) varies between 0.036% (360 ppm) and 0.041% (412 ppm), depending on the location.
In humans, exposure to at concentrations greater than 5% causes the development of hypercapnia
Hypercapnia (from the Greek ''hyper'', "above" or "too much" and ''kapnos'', "smoke"), also known as hypercarbia and CO2 retention, is a condition of abnormally elevated carbon dioxide (CO2) levels in the blood. Carbon dioxide is a gaseous pro ...
and respiratory acidosis
The respiratory system (also respiratory apparatus, ventilatory system) is a biological system consisting of specific organs and structures used for gas exchange in animals and plants. The anatomy and physiology that make this happen varies gr ...
.[ Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License] Concentrations of 7% to 10% (70,000 to 100,000 ppm) may cause suffocation, even in the presence of sufficient oxygen, manifesting as dizziness, headache, visual and hearing dysfunction, and unconsciousness within a few minutes to an hour.Goma
Goma is a city in the eastern Democratic Republic of the Congo. It is the Capital city, capital and largest city of the North Kivu, North Kivu Province; it is located on the northern shore of Lake Kivu and shares borders with the Bukumu Chiefdo ...
by emissions from the nearby volcano Mount Nyiragongo. The Swahili term for this phenomenon is .
 Adaptation to increased concentrations of occurs in humans, including modified breathing and kidney bicarbonate production, in order to balance the effects of blood acidification ( acidosis). Several studies suggested that 2.0 percent inspired concentrations could be used for closed air spaces (e.g. a
Adaptation to increased concentrations of occurs in humans, including modified breathing and kidney bicarbonate production, in order to balance the effects of blood acidification ( acidosis). Several studies suggested that 2.0 percent inspired concentrations could be used for closed air spaces (e.g. a submarine
A submarine (often shortened to sub) is a watercraft capable of independent operation underwater. (It differs from a submersible, which has more limited underwater capability.) The term "submarine" is also sometimes used historically or infor ...
) since the adaptation is physiological and reversible, as deterioration in performance or in normal physical activity does not happen at this level of exposure for five days. Yet, other studies show a decrease in cognitive function even at much lower levels.
Below 1%
There are few studies of the health effects of long-term continuous exposure on humans and animals at levels below 1%. Occupational exposure limits have been set in the United States at 0.5% (5000 ppm) for an eight-hour period.International Space Station
The International Space Station (ISS) is a large space station that was Assembly of the International Space Station, assembled and is maintained in low Earth orbit by a collaboration of five space agencies and their contractors: NASA (United ...
crew experienced headaches, lethargy, mental slowness, emotional irritation, and sleep disruption. Studies in animals at 0.5% have demonstrated kidney calcification and bone loss after eight weeks of exposure. A study of humans exposed in 2.5 hour sessions demonstrated significant negative effects on cognitive abilities at concentrations as low as 0.1% (1000ppm) likely due to induced increases in cerebral blood flow.
Ventilation
 Poor ventilation is one of the main causes of excessive concentrations in closed spaces, leading to poor indoor air quality. Carbon dioxide differential above outdoor concentrations at steady state conditions (when the occupancy and ventilation system operation are sufficiently long that concentration has stabilized) are sometimes used to estimate ventilation rates per person. Higher concentrations are associated with occupant health, comfort and performance degradation.
Poor ventilation is one of the main causes of excessive concentrations in closed spaces, leading to poor indoor air quality. Carbon dioxide differential above outdoor concentrations at steady state conditions (when the occupancy and ventilation system operation are sufficiently long that concentration has stabilized) are sometimes used to estimate ventilation rates per person. Higher concentrations are associated with occupant health, comfort and performance degradation. ASHRAE
The American Society of Heating, Refrigerating and Air-Conditioning Engineers (ASHRAE ) is an American professional association seeking to advance heating, ventilation, air conditioning and refrigeration (HVAC&R) systems design and constructio ...
Standard 62.1–2007 ventilation rates may result in indoor concentrations up to 2,100 ppm above ambient outdoor conditions. Thus if the outdoor concentration is 400 ppm, indoor concentrations may reach 2,500 ppm with ventilation rates that meet this industry consensus standard. Concentrations in poorly ventilated spaces can be found even higher than this (range of 3,000 or 4,000 ppm).
Miners, who are particularly vulnerable to gas exposure due to insufficient ventilation, referred to mixtures of carbon dioxide and nitrogen as " blackdamp", "choke damp" or "stythe". Before more effective technologies were developed, miners
A miner is a person who extracts ore, coal, chalk, clay, or other minerals from the earth through mining. There are two senses in which the term is used. In its narrowest sense, a miner is someone who works at the rock face (mining), face; cutt ...
would frequently monitor for dangerous levels of blackdamp and other gases in mine shafts by bringing a caged canary with them as they worked. The canary is more sensitive to asphyxiant gases than humans, and as it became unconscious would stop singing and fall off its perch. The Davy lamp could also detect high levels of blackdamp (which sinks, and collects near the floor) by burning less brightly, while methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes ...
, another suffocating gas and explosion risk, would make the lamp burn more brightly.
In February 2020, three people died from suffocation at a party in Moscow when dry ice (frozen ) was added to a swimming pool to cool it down. A similar accident occurred in 2018 when a woman died from fumes emanating from the large amount of dry ice she was transporting in her car.
Indoor air
Humans spend more and more time in a confined atmosphere (around 80-90% of the time in a building or vehicle). According to the French Agency for Food, Environmental and Occupational Health & Safety (ANSES) and various actors in France, the rate in the indoor air of buildings (linked to human or animal occupancy and the presence of combustion
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel (the reductant) and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion ...
installations), weighted by air renewal, is "usually between about 350 and 2,500 ppm".
In homes, schools, nurseries and offices, there are no systematic relationships between the levels of and other pollutants, and indoor is statistically not a good predictor of pollutants linked to outdoor road (or air, etc.) traffic. is the parameter that changes the fastest (with hygrometry and oxygen levels when humans or animals are gathered in a closed or poorly ventilated room). In poor countries, many open hearths are sources of and CO emitted directly into the living environment.
Outdoor areas with elevated concentrations
Local concentrations of carbon dioxide can reach high values near strong sources, especially those that are isolated by surrounding terrain. At the Bossoleto hot spring near Rapolano Terme in Tuscany
Tuscany ( ; ) is a Regions of Italy, region in central Italy with an area of about and a population of 3,660,834 inhabitants as of 2025. The capital city is Florence.
Tuscany is known for its landscapes, history, artistic legacy, and its in ...
, Italy, situated in a bowl-shaped depression about in diameter, concentrations of rise to above 75% overnight, sufficient to kill insects and small animals. After sunrise the gas is dispersed by convection. High concentrations of produced by disturbance of deep lake water saturated with are thought to have caused 37 fatalities at Lake Monoun, Cameroon
Cameroon, officially the Republic of Cameroon, is a country in Central Africa. It shares boundaries with Nigeria to the west and north, Chad to the northeast, the Central African Republic to the east, and Equatorial Guinea, Gabon, and the R ...
in 1984 and 1700 casualties at Lake Nyos, Cameroon in 1986.
Human physiology
Content
The body produces approximately of carbon dioxide per day per person, containing of carbon. In humans, this carbon dioxide is carried through the venous system and is breathed out through the lungs, resulting in lower concentrations in the arteries. The carbon dioxide content of the blood is often given as the partial pressure
In a mixture of gases, each constituent gas has a partial pressure which is the notional pressure of that constituent gas as if it alone occupied the entire volume of the original mixture at the same temperature. The total pressure of an ideal g ...
, which is the pressure which carbon dioxide would have had if it alone occupied the volume. In humans, the blood carbon dioxide contents are shown in the adjacent table.
Transport in the blood
is carried in blood in three different ways. Exact percentages vary between arterial and venous blood.
* Majority (about 70% to 80%) is converted to bicarbonate
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula .
Bicarbonate serves a crucial bioche ...
ions by the enzyme carbonic anhydrase in the red blood cells,blood plasma
Blood plasma is a light Amber (color), amber-colored liquid component of blood in which blood cells are absent, but which contains Blood protein, proteins and other constituents of whole blood in Suspension (chemistry), suspension. It makes up ...
hemoglobin
Hemoglobin (haemoglobin, Hb or Hgb) is a protein containing iron that facilitates the transportation of oxygen in red blood cells. Almost all vertebrates contain hemoglobin, with the sole exception of the fish family Channichthyidae. Hemoglobin ...
as carbamino compoundsHemoglobin
Hemoglobin (haemoglobin, Hb or Hgb) is a protein containing iron that facilitates the transportation of oxygen in red blood cells. Almost all vertebrates contain hemoglobin, with the sole exception of the fish family Channichthyidae. Hemoglobin ...
, the main oxygen-carrying molecule in red blood cells, carries both oxygen and carbon dioxide. However, the bound to hemoglobin does not bind to the same site as oxygen. Instead, it combines with the N-terminal groups on the four globin chains. However, because of allosteric effects on the hemoglobin molecule, the binding of decreases the amount of oxygen that is bound for a given partial pressure of oxygen. This is known as the Haldane Effect, and is important in the transport of carbon dioxide from the tissues to the lungs. Conversely, a rise in the partial pressure of or a lower pH will cause offloading of oxygen from hemoglobin, which is known as the Bohr effect.
Regulation of respiration
Carbon dioxide is one of the mediators of local autoregulation of blood supply. If its concentration is high, the capillaries expand to allow a greater blood flow to that tissue.
Bicarbonate ions are crucial for regulating blood pH. A person's breathing rate influences the level of in their blood. Breathing that is too slow or shallow causes respiratory acidosis
The respiratory system (also respiratory apparatus, ventilatory system) is a biological system consisting of specific organs and structures used for gas exchange in animals and plants. The anatomy and physiology that make this happen varies gr ...
, while breathing that is too rapid leads to hyperventilation
Hyperventilation is irregular breathing that occurs when the rate or tidal volume of breathing eliminates more carbon dioxide than the body can produce. This leads to hypocapnia, a reduced concentration of carbon dioxide dissolved in the blo ...
, which can cause respiratory alkalosis.
Although the body requires oxygen for metabolism, low oxygen levels normally do not stimulate breathing. Rather, breathing is stimulated by higher carbon dioxide levels. As a result, breathing low-pressure air or a gas mixture with no oxygen at all (such as pure nitrogen) can lead to loss of consciousness without ever experiencing air hunger. This is especially perilous for high-altitude fighter pilots. It is also why flight attendants instruct passengers, in case of loss of cabin pressure, to apply the oxygen mask
An oxygen mask is a mask that provides a method to transfer breathing gas, breathing oxygen gas from a storage tank to the lungs. Oxygen masks may cover only the nose and mouth (oral nasal mask) or the entire face (full-face mask). They may be ma ...
to themselves first before helping others; otherwise, one risks losing consciousness.
Concentrations and role in the environment
Atmosphere

Oceans
Ocean acidification
Carbon dioxide dissolves in the ocean to form carbonic acid (), bicarbonate (), and carbonate (). There is about fifty times as much carbon dioxide dissolved in the oceans as exists in the atmosphere. The oceans act as an enormous carbon sink
A carbon sink is a natural or artificial carbon sequestration process that "removes a greenhouse gas, an aerosol or a precursor of a greenhouse gas from the atmosphere". These sinks form an important part of the natural carbon cycle. An overar ...
, and have taken up about a third of emitted by human activity.

Hydrothermal vents
Carbon dioxide is also introduced into the oceans through hydrothermal vents. The ''Champagne'' hydrothermal vent, found at the Northwest Eifuku volcano in the Mariana Trench
The Mariana Trench is an oceanic trench located in the western Pacific Ocean, about east of the Mariana Islands; it is the deep sea, deepest oceanic trench on Earth. It is crescent-shaped and measures about in length and in width. The maxi ...
, produces almost pure liquid carbon dioxide, one of only two known sites in the world as of 2004, the other being in the Okinawa Trough. The finding of a submarine lake of liquid carbon dioxide in the Okinawa Trough was reported in 2006.
Sources
The burning of fossil fuels for energy produces 36.8 billion tonnes of per year as of 2023. Nearly all of this goes into the atmosphere, where approximately half is subsequently absorbed into natural carbon sink
A carbon sink is a natural or artificial carbon sequestration process that "removes a greenhouse gas, an aerosol or a precursor of a greenhouse gas from the atmosphere". These sinks form an important part of the natural carbon cycle. An overar ...
s. Less than 1% of produced annually is put to commercial use.
Biological processes
Carbon dioxide is a by-product of the fermentation
Fermentation is a type of anaerobic metabolism which harnesses the redox potential of the reactants to make adenosine triphosphate (ATP) and organic end products. Organic molecules, such as glucose or other sugars, are catabolized and reduce ...
of sugar in the brewing
Brewing is the production of beer by steeping a starch source (commonly cereal grains, the most popular of which is barley) in water and #Fermenting, fermenting the resulting sweet liquid with Yeast#Beer, yeast. It may be done in a brewery ...
of beer
Beer is an alcoholic beverage produced by the brewing and fermentation of starches from cereal grain—most commonly malted barley, although wheat, maize (corn), rice, and oats are also used. The grain is mashed to convert starch in the ...
, whisky
Whisky or whiskey is a type of liquor made from Fermentation in food processing, fermented grain mashing, mash. Various grains (which may be Malting, malted) are used for different varieties, including barley, Maize, corn, rye, and wheat. Whisky ...
and other alcoholic beverage
Drinks containing alcohol (drug), alcohol are typically divided into three classes—beers, wines, and Distilled beverage, spirits—with alcohol content typically between 3% and 50%. Drinks with less than 0.5% are sometimes considered Non-al ...
s and in the production of bioethanol. Yeast
Yeasts are eukaryotic, single-celled microorganisms classified as members of the fungus kingdom (biology), kingdom. The first yeast originated hundreds of millions of years ago, and at least 1,500 species are currently recognized. They are est ...
metabolizes sugar to produce and ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the ps ...
, also known as alcohol, as follows:
:
All aerobic organisms produce when they oxidize carbohydrate
A carbohydrate () is a biomolecule composed of carbon (C), hydrogen (H), and oxygen (O) atoms. The typical hydrogen-to-oxygen atomic ratio is 2:1, analogous to that of water, and is represented by the empirical formula (where ''m'' and ''n'' ...
s, fatty acid
In chemistry, in particular in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated and unsaturated compounds#Organic chemistry, saturated or unsaturated. Most naturally occurring fatty acids have an ...
s, and protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
s. The large number of reactions involved are exceedingly complex and not described easily. Refer to cellular respiration
Cellular respiration is the process of oxidizing biological fuels using an inorganic electron acceptor, such as oxygen, to drive production of adenosine triphosphate (ATP), which stores chemical energy in a biologically accessible form. Cell ...
, anaerobic respiration and photosynthesis
Photosynthesis ( ) is a system of biological processes by which photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical energy necessary to fuel their metabo ...
. The equation for the respiration of glucose and other monosaccharide
Monosaccharides (from Greek '' monos'': single, '' sacchar'': sugar), also called simple sugars, are the simplest forms of sugar and the most basic units (monomers) from which all carbohydrates are built.
Chemically, monosaccharides are polyhy ...
s is:
:
Anaerobic organisms decompose organic material producing methane and carbon dioxide together with traces of other compounds. Regardless of the type of organic material, the production of gases follows well defined kinetic pattern. Carbon dioxide comprises about 40–45% of the gas that emanates from decomposition in landfills (termed " landfill gas"). Most of the remaining 50–55% is methane.
Combustion
The combustion
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel (the reductant) and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion ...
of all carbon-based fuel Carbon-based may refer to:
* Biology
* based on Carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to fo ...
s, such as methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes ...
(natural gas
Natural gas (also fossil gas, methane gas, and gas) is a naturally occurring compound of gaseous hydrocarbons, primarily methane (95%), small amounts of higher alkanes, and traces of carbon dioxide and nitrogen, hydrogen sulfide and helium ...
), petroleum distillates (gasoline
Gasoline ( North American English) or petrol ( Commonwealth English) is a petrochemical product characterized as a transparent, yellowish, and flammable liquid normally used as a fuel for spark-ignited internal combustion engines. When for ...
, diesel, kerosene
Kerosene, or paraffin, is a combustibility, combustible hydrocarbon liquid which is derived from petroleum. It is widely used as a fuel in Aviation fuel, aviation as well as households. Its name derives from the Greek (''kērós'') meaning " ...
, propane
Propane () is a three-carbon chain alkane with the molecular formula . It is a gas at standard temperature and pressure, but becomes liquid when compressed for transportation and storage. A by-product of natural gas processing and petroleum ref ...
), coal, wood and generic organic matter produces carbon dioxide and, except in the case of pure carbon, water. As an example, the chemical reaction between methane and oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
:
:
Iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
is reduced from its oxides with coke in a blast furnace
A blast furnace is a type of metallurgical furnace used for smelting to produce industrial metals, generally pig iron, but also others such as lead or copper. ''Blast'' refers to the combustion air being supplied above atmospheric pressure.
In a ...
, producing pig iron
Pig iron, also known as crude iron, is an intermediate good used by the iron industry in the production of steel. It is developed by smelting iron ore in a blast furnace. Pig iron has a high carbon content, typically 3.8–4.7%, along with si ...
and carbon dioxide:
:
By-product from hydrogen production
Carbon dioxide is a byproduct of the industrial production of hydrogen by steam reforming
Steam reforming or steam methane reforming (SMR) is a method for producing syngas (hydrogen and carbon monoxide) by reaction of hydrocarbons with water. Commonly, natural gas is the feedstock. The main purpose of this technology is often hydrogen ...
and the water gas shift reaction in ammonia production. These processes begin with the reaction of water and natural gas (mainly methane).
Thermal decomposition of limestone
It is produced by thermal decomposition of limestone, by heating ( calcining) at about , in the manufacture of quicklime ( calcium oxide, CaO), a compound that has many industrial uses:
:
Acids liberate from most metal carbonates. Consequently, it may be obtained directly from natural carbon dioxide springs, where it is produced by the action of acidified water on limestone
Limestone is a type of carbonate rock, carbonate sedimentary rock which is the main source of the material Lime (material), lime. It is composed mostly of the minerals calcite and aragonite, which are different Polymorphism (materials science) ...
or dolomite. The reaction between hydrochloric acid
Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungency, pungent smell. It is classified as a acid strength, strong acid. It is ...
and calcium carbonate (limestone or chalk) is shown below:
:
The carbonic acid
Carbonic acid is a chemical compound with the chemical formula . The molecule rapidly converts to water and carbon dioxide in the presence of water. However, in the absence of water, it is quite stable at room temperature. The interconversion ...
() then decomposes to water and :
:
Such reactions are accompanied by foaming or bubbling, or both, as the gas is released. They have widespread uses in industry because they can be used to neutralize waste acid streams.
Commercial uses
 Around 230 Mt of are used each year, mostly in the fertiliser industry for urea production (130 million tonnes) and in the oil and gas industry for enhanced oil recovery (70 to 80 million tonnes).
Around 230 Mt of are used each year, mostly in the fertiliser industry for urea production (130 million tonnes) and in the oil and gas industry for enhanced oil recovery (70 to 80 million tonnes).[ Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License] However, the potential to use products is very small compared to the total volume of that could foreseeably be captured.[ Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License] The vast majority of captured is considered a waste product and sequestered in underground geologic formations.[Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License]
Precursor to chemicals
In the chemical industry, carbon dioxide is mainly consumed as an ingredient in the production of urea
Urea, also called carbamide (because it is a diamide of carbonic acid), is an organic compound with chemical formula . This amide has two Amine, amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest am ...
, with a smaller fraction being used to produce methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical compound and the simplest aliphatic Alcohol (chemistry), alcohol, with the chemical formula (a methyl group linked to a hydroxyl group, often ab ...
and a range of other products. Some carboxylic acid derivatives such as sodium salicylate are prepared using by the Kolbe–Schmitt reaction.
Captured could be to produce methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical compound and the simplest aliphatic Alcohol (chemistry), alcohol, with the chemical formula (a methyl group linked to a hydroxyl group, often ab ...
or electrofuels. To be carbon-neutral, the would need to come from bioenergy production or direct air capture.[IEA (2020), ]
CCUS in Clean Energy Transitions
', IEA, Paris Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License
Fossil fuel recovery
Carbon dioxide is used in enhanced oil recovery where it is injected into or adjacent to producing oil wells, usually under supercritical conditions, when it becomes miscible with the oil. This approach can increase original oil recovery by reducing residual oil saturation by 7–23% additional to primary extraction. It acts as both a pressurizing agent and, when dissolved into the underground crude oil, significantly reduces its viscosity, and changing surface chemistry enabling the oil to flow more rapidly through the reservoir to the removal well.
Most injected in -EOR projects comes from naturally occurring underground deposits.[ Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License] Some used in EOR is captured from industrial facilities such as natural gas processing plants, using carbon capture technology and transported to the oilfield in pipelines.
Agriculture
Plants require carbon dioxide to conduct photosynthesis. The atmospheres of greenhouses may (if of large size, must) be enriched with additional to sustain and increase the rate of plant growth. At very high concentrations (100 times atmospheric concentration, or greater), carbon dioxide can be toxic to animal life, so raising the concentration to 10,000 ppm (1%) or higher for several hours will eliminate pests such as whiteflies and spider mites in a greenhouse. Some plants respond more favorably to rising carbon dioxide concentrations than others, which can lead to vegetation regime shifts like woody plant encroachment.
Foods
 Carbon dioxide is a
Carbon dioxide is a food additive
Food additives are substances added to food to preserve flavor or enhance taste, appearance, or other sensory qualities. Some additives, such as vinegar ( pickling), salt ( salting), smoke ( smoking) and sugar ( crystallization), have been used f ...
used as a propellant and acidity regulator in the food industry. It is approved for usage in the EU (listed as E number
E numbers, short for Europe numbers, are codes for substances used as food additives, including those found naturally in many foods, such as vitamin C, for use within the European Union (EU) and European Free Trade Association (EFTA). Commonly ...
E290), US, Australia and New Zealand (listed by its INS number 290).
A candy called Pop Rocks is pressurized with carbon dioxide gas at about . When placed in the mouth, it dissolves (just like other hard candy) and releases the gas bubbles with an audible pop.
Leavening agents cause dough to rise by producing carbon dioxide. Baker's yeast produces carbon dioxide by fermentation of sugars within the dough, while chemical leaveners such as baking powder and baking soda
Sodium bicarbonate (IUPAC name: sodium hydrogencarbonate), commonly known as baking soda or bicarbonate of soda (or simply “bicarb” especially in the UK) is a chemical compound with the formula NaHCO3. It is a salt (chemistry), salt compose ...
release carbon dioxide when heated or if exposed to acid
An acid is a molecule or ion capable of either donating a proton (i.e. Hydron, hydrogen cation, H+), known as a Brønsted–Lowry acid–base theory, Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis ...
s.
Beverages
Carbon dioxide is used to produce carbonated soft drink
A soft drink (see #Terminology, § Terminology for other names) is a class of non-alcoholic drink, usually (but not necessarily) Carbonated water, carbonated, and typically including added Sweetness, sweetener. Flavors used to be Natural flav ...
s and soda water. Traditionally, the carbonation of beer and sparkling wine came about through natural fermentation, but many manufacturers carbonate these drinks with carbon dioxide recovered from the fermentation process. In the case of bottled and kegged beer, the most common method used is carbonation with recycled carbon dioxide. With the exception of British real ale, draught beer is usually transferred from kegs in a cold room or cellar to dispensing taps on the bar using pressurized carbon dioxide, sometimes mixed with nitrogen.
The taste of soda water (and related taste sensations in other carbonated beverages) is an effect of the dissolved carbon dioxide rather than the bursting bubbles of the gas. Carbonic anhydrase 4 converts carbon dioxide to carbonic acid
Carbonic acid is a chemical compound with the chemical formula . The molecule rapidly converts to water and carbon dioxide in the presence of water. However, in the absence of water, it is quite stable at room temperature. The interconversion ...
leading to a sour
The gustatory system or sense of taste is the sensory system that is partially responsible for the perception of taste. Taste is the perception stimulated when a substance in the mouth biochemistry, reacts chemically with taste receptor cells l ...
taste, and also the dissolved carbon dioxide induces a somatosensory response.
Winemaking
 Carbon dioxide in the form of
Carbon dioxide in the form of dry ice
Dry ice is the solid form of carbon dioxide. It is commonly used for temporary refrigeration as CO2 does not have a liquid state at normal atmospheric pressure and Sublimation (phase transition), sublimes directly from the solid state to the gas ...
is often used during the cold soak phase in winemaking
Winemaking, wine-making, or vinification is the production of wine, starting with the selection of the fruit, its Ethanol fermentation, fermentation into alcohol, and the bottling of the finished liquid. The history of wine-making stretches over ...
to cool clusters of grape
A grape is a fruit, botanically a berry, of the deciduous woody vines of the flowering plant genus ''Vitis''. Grapes are a non- climacteric type of fruit, generally occurring in clusters.
The cultivation of grapes began approximately 8,0 ...
s quickly after picking to help prevent spontaneous fermentation
Fermentation is a type of anaerobic metabolism which harnesses the redox potential of the reactants to make adenosine triphosphate (ATP) and organic end products. Organic molecules, such as glucose or other sugars, are catabolized and reduce ...
by wild yeast
Yeasts are eukaryotic, single-celled microorganisms classified as members of the fungus kingdom (biology), kingdom. The first yeast originated hundreds of millions of years ago, and at least 1,500 species are currently recognized. They are est ...
. The main advantage of using dry ice over water ice is that it cools the grapes without adding any additional water that might decrease the sugar concentration in the grape must, and thus the alcohol
Alcohol may refer to:
Common uses
* Alcohol (chemistry), a class of compounds
* Ethanol, one of several alcohols, commonly known as alcohol in everyday life
** Alcohol (drug), intoxicant found in alcoholic beverages
** Alcoholic beverage, an alco ...
concentration in the finished wine. Carbon dioxide is also used to create a hypoxic environment for carbonic maceration, the process used to produce Beaujolais wine.
Carbon dioxide is sometimes used to top up wine bottles or other storage vessels such as barrels to prevent oxidation, though it has the problem that it can dissolve into the wine, making a previously still wine slightly fizzy. For this reason, other gases such as nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
or argon
Argon is a chemical element; it has symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abu ...
are preferred for this process by professional wine makers.
Stunning animals
Carbon dioxide is often used to "stun" animals before slaughter. "Stunning" may be a misnomer, as the animals are not knocked out immediately and may suffer distress.
Inert gas
Carbon dioxide is one of the most commonly used compressed gases for pneumatic (pressurized gas) systems in portable pressure tools. Carbon dioxide is also used as an atmosphere for welding
Welding is a fabrication (metal), fabrication process that joins materials, usually metals or thermoplastics, primarily by using high temperature to melting, melt the parts together and allow them to cool, causing Fusion welding, fusion. Co ...
, although in the welding arc, it reacts to oxidize most metals. Use in the automotive industry is common despite significant evidence that welds made in carbon dioxide are more brittle
A material is brittle if, when subjected to stress, it fractures with little elastic deformation and without significant plastic deformation. Brittle materials absorb relatively little energy prior to fracture, even those of high strength. ...
than those made in more inert atmospheres. When used for MIG welding, use is sometimes referred to as MAG welding, for Metal Active Gas, as can react at these high temperatures. It tends to produce a hotter puddle than truly inert atmospheres, improving the flow characteristics. Although, this may be due to atmospheric reactions occurring at the puddle site. This is usually the opposite of the desired effect when welding, as it tends to embrittle the site, but may not be a problem for general mild steel welding, where ultimate ductility is not a major concern.
Carbon dioxide is used in many consumer products that require pressurized gas because it is inexpensive and nonflammable, and because it undergoes a phase transition from gas to liquid at room temperature at an attainable pressure of approximately , allowing far more carbon dioxide to fit in a given container than otherwise would. Life jackets often contain canisters of pressured carbon dioxide for quick inflation. Aluminium
Aluminium (or aluminum in North American English) is a chemical element; it has chemical symbol, symbol Al and atomic number 13. It has a density lower than that of other common metals, about one-third that of steel. Aluminium has ...
capsules of are also sold as supplies of compressed gas for air guns, paintball
Paintball is a competitive sport, competitive team sport, team shooting sport in which players eliminate opponents from play by hitting them with spherical dye-filled gelatin capsules called Paintball equipment#Paintballs, paintballs that b ...
markers/guns, inflating bicycle tires, and for making carbonated water
Carbonated water is water containing dissolved carbon dioxide gas, either artificially injected under pressure, or occurring due to natural geological processes. Carbonation causes small bubbles to form, giving the water an effervescent quali ...
. High concentrations of carbon dioxide can also be used to kill pests. Liquid carbon dioxide is used in supercritical drying
Supercritical drying, also known as critical point drying, is a process to remove liquid in a precise and controlled way. It is useful in the production of microelectromechanical systems (MEMS), the drying of spices, the production of aerogel ...
of some food products and technological materials, in the preparation of specimens for scanning electron microscopy
A scanning electron microscope (SEM) is a type of electron microscope that produces images of a sample by scanning the surface with a focused beam of electrons. The electrons interact with atoms in the sample, producing various signals that ...
Fire extinguisher
 Carbon dioxide can be used to extinguish flames by flooding the environment around the flame with the gas. It does not itself react to extinguish the flame, but starves the flame of oxygen by displacing it. Some fire extinguishers, especially those designed for electrical fires, contain liquid carbon dioxide under pressure. Carbon dioxide extinguishers work well on small flammable liquid and electrical fires, but not on ordinary combustible fires, because they do not cool the burning substances significantly, and when the carbon dioxide disperses, they can catch fire upon exposure to atmospheric oxygen. They are mainly used in server rooms.
Carbon dioxide has also been widely used as an extinguishing agent in fixed fire-protection systems for local application of specific hazards and total flooding of a protected space.
Carbon dioxide can be used to extinguish flames by flooding the environment around the flame with the gas. It does not itself react to extinguish the flame, but starves the flame of oxygen by displacing it. Some fire extinguishers, especially those designed for electrical fires, contain liquid carbon dioxide under pressure. Carbon dioxide extinguishers work well on small flammable liquid and electrical fires, but not on ordinary combustible fires, because they do not cool the burning substances significantly, and when the carbon dioxide disperses, they can catch fire upon exposure to atmospheric oxygen. They are mainly used in server rooms.
Carbon dioxide has also been widely used as an extinguishing agent in fixed fire-protection systems for local application of specific hazards and total flooding of a protected space. International Maritime Organization
The International Maritime Organization (IMO; ; ) is a List of specialized agencies of the United Nations, specialized agency of the United Nations responsible for regulating maritime transport. The IMO was established following agreement at a ...
standards recognize carbon dioxide systems for fire protection of ship holds and engine rooms. Carbon dioxide-based fire-protection systems have been linked to several deaths, because it can cause suffocation in sufficiently high concentrations. A review of systems identified 51 incidents between 1975 and the date of the report (2000), causing 72 deaths and 145 injuries.
Supercritical as solvent
Liquid carbon dioxide is a good solvent
A solvent (from the Latin language, Latin ''wikt:solvo#Latin, solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a Solution (chemistry), solution. A solvent is usually a liquid but can also be a solid, a gas ...
for many lipophilic
Lipophilicity (from Greek language, Greek λίπος "fat" and :wikt:φίλος, φίλος "friendly") is the ability of a chemical compound to dissolve in fats, oils, lipids, and non-polar solvents such as hexane or toluene. Such compounds are c ...
organic compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-co ...
s and is used to decaffeinate coffee
Coffee is a beverage brewed from roasted, ground coffee beans. Darkly colored, bitter, and slightly acidic, coffee has a stimulating effect on humans, primarily due to its caffeine content, but decaffeinated coffee is also commercially a ...
.
Refrigerant
 Liquid and solid carbon dioxide are important
Liquid and solid carbon dioxide are important refrigerant
A refrigerant is a working fluid used in the cooling, heating, or reverse cooling/heating cycles of air conditioning systems and heat pumps, where they undergo a repeated phase transition from a liquid to a gas and back again. Refrigerants are ...
s, especially in the food industry, where they are employed during the transportation and storage of ice cream and other frozen foods. Solid carbon dioxide is called "dry ice" and is used for small shipments where refrigeration equipment is not practical. Solid carbon dioxide is always below at regular atmospheric pressure, regardless of the air temperature.
Liquid carbon dioxide (industry nomenclature R744 or R-744) was used as a refrigerant prior to the use of dichlorodifluoromethane (R12, a chlorofluorocarbon
Chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) are fully or partly Halogenation, halogenated hydrocarbons that contain carbon (C), hydrogen (H), chlorine (Cl), and fluorine (F). They are produced as volatility (chemistry), volat ...
(CFC) compound). might enjoy a renaissance because one of the main substitutes to CFCs, 1,1,1,2-tetrafluoroethane
1,1,1,2-Tetrafluoroethane (also known as norflurane ( INN), R-134a, Klea 134a, Freon 134a, Forane 134a, Genetron 134a, Green Gas, Florasol 134a, Suva 134a, HFA-134a, or HFC-134a) is a hydrofluorocarbon (HFC) and haloalkane refrigerant with th ...
( R134a, a hydrofluorocarbon (HFC) compound) contributes to climate change
Present-day climate change includes both global warming—the ongoing increase in Global surface temperature, global average temperature—and its wider effects on Earth's climate system. Climate variability and change, Climate change in ...
more than does. physical properties are highly favorable for cooling, refrigeration, and heating purposes, having a high volumetric cooling capacity. Due to the need to operate at pressures of up to , systems require highly mechanically resistant reservoirs and components that have already been developed for mass production in many sectors. In automobile air conditioning, in more than 90% of all driving conditions for latitudes higher than 50°, (R744) operates more efficiently than systems using HFCs (e.g., R134a). Its environmental advantages ( GWP of 1, non-ozone depleting, non-toxic, non-flammable) could make it the future working fluid to replace current HFCs in cars, supermarkets, and heat pump water heaters, among others. Coca-Cola
Coca-Cola, or Coke, is a cola soft drink manufactured by the Coca-Cola Company. In 2013, Coke products were sold in over 200 countries and territories worldwide, with consumers drinking more than 1.8 billion company beverage servings ...
has fielded -based beverage coolers and the U.S. Army is interested in refrigeration and heating technology.
Minor uses
 Carbon dioxide is the lasing medium in a carbon-dioxide laser, which is one of the earliest type of lasers.
Carbon dioxide can be used as a means of controlling the pH of swimming pools, by continuously adding gas to the water, thus keeping the pH from rising. Among the advantages of this is the avoidance of handling (more hazardous) acids. Similarly, it is also used in the maintaining reef aquaria, where it is commonly used in calcium reactors to temporarily lower the pH of water being passed over
Carbon dioxide is the lasing medium in a carbon-dioxide laser, which is one of the earliest type of lasers.
Carbon dioxide can be used as a means of controlling the pH of swimming pools, by continuously adding gas to the water, thus keeping the pH from rising. Among the advantages of this is the avoidance of handling (more hazardous) acids. Similarly, it is also used in the maintaining reef aquaria, where it is commonly used in calcium reactors to temporarily lower the pH of water being passed over calcium carbonate
Calcium carbonate is a chemical compound with the chemical formula . It is a common substance found in Rock (geology), rocks as the minerals calcite and aragonite, most notably in chalk and limestone, eggshells, gastropod shells, shellfish skel ...
in order to allow the calcium carbonate to dissolve into the water more freely, where it is used by some coral
Corals are colonial marine invertebrates within the subphylum Anthozoa of the phylum Cnidaria. They typically form compact Colony (biology), colonies of many identical individual polyp (zoology), polyps. Coral species include the important Coral ...
s to build their skeleton.
Used as the primary coolant in the British advanced gas-cooled reactor for nuclear power generation.
Carbon dioxide induction is commonly used for the euthanasia of laboratory research animals. Methods to administer include placing animals directly into a closed, prefilled chamber containing , or exposure to a gradually increasing concentration of . The American Veterinary Medical Association
The American Veterinary Medical Association (AVMA) is an American not-for-profit association founded in 1863 that represents more than 105,000 veterinarians.
The AVMA provides information resources, continuing education opportunities, publicat ...
's 2020 guidelines for carbon dioxide induction state that a displacement rate of 30–70% of the chamber or cage volume per minute is optimal for the humane euthanasia of small rodents.
History of discovery
 Carbon dioxide was the first gas to be described as a discrete substance. In about 1640, the Flemish chemist
Carbon dioxide was the first gas to be described as a discrete substance. In about 1640, the Flemish chemist Jan Baptist van Helmont
Jan Baptist van Helmont ( , ; 12 January 1580 – 30 December 1644) was a chemist, physiologist, and physician from Brussels. He worked during the years just after Paracelsus and the rise of iatrochemistry, and is sometimes considered to be ...
observed that when he burned charcoal in a closed vessel, the mass of the resulting ash was much less than that of the original charcoal. His interpretation was that the rest of the charcoal had been transmuted into an invisible substance he termed a "gas" (from Greek "chaos") or "wild spirit" (''spiritus sylvestris'').
The properties of carbon dioxide were further studied in the 1750s by the Scottish physician Joseph Black. He found that limestone
Limestone is a type of carbonate rock, carbonate sedimentary rock which is the main source of the material Lime (material), lime. It is composed mostly of the minerals calcite and aragonite, which are different Polymorphism (materials science) ...
(calcium carbonate
Calcium carbonate is a chemical compound with the chemical formula . It is a common substance found in Rock (geology), rocks as the minerals calcite and aragonite, most notably in chalk and limestone, eggshells, gastropod shells, shellfish skel ...
) could be heated or treated with acid
An acid is a molecule or ion capable of either donating a proton (i.e. Hydron, hydrogen cation, H+), known as a Brønsted–Lowry acid–base theory, Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis ...
s to yield a gas he called "fixed air". He observed that the fixed air was denser than air and supported neither flame nor animal life. Black also found that when bubbled through limewater (a saturated aqueous solution of calcium hydroxide
Calcium hydroxide (traditionally called slaked lime) is an inorganic compound with the chemical formula Ca( OH)2. It is a colorless crystal or white powder and is produced when quicklime ( calcium oxide) is mixed with water. Annually, approxim ...
), it would precipitate calcium carbonate. He used this phenomenon to illustrate that carbon dioxide is produced by animal respiration and microbial fermentation. In 1772, English chemist Joseph Priestley
Joseph Priestley (; 24 March 1733 – 6 February 1804) was an English chemist, Unitarian, Natural philosophy, natural philosopher, English Separatist, separatist theologian, Linguist, grammarian, multi-subject educator and Classical libera ...
published a paper entitled ''Impregnating Water with Fixed Air'' in which he described a process of dripping sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
(or ''oil of vitriol'' as Priestley knew it) on chalk in order to produce carbon dioxide, and forcing the gas to dissolve by agitating a bowl of water in contact with the gas.Humphry Davy
Sir Humphry Davy, 1st Baronet (17 December 177829 May 1829) was a British chemist and inventor who invented the Davy lamp and a very early form of arc lamp. He is also remembered for isolating, by using electricity, several Chemical element, e ...
and Michael Faraday
Michael Faraday (; 22 September 1791 – 25 August 1867) was an English chemist and physicist who contributed to the study of electrochemistry and electromagnetism. His main discoveries include the principles underlying electromagnetic inducti ...
.dry ice
Dry ice is the solid form of carbon dioxide. It is commonly used for temporary refrigeration as CO2 does not have a liquid state at normal atmospheric pressure and Sublimation (phase transition), sublimes directly from the solid state to the gas ...
) was given by the French inventor Adrien-Jean-Pierre Thilorier, who in 1835 opened a pressurized container of liquid carbon dioxide, only to find that the cooling produced by the rapid evaporation of the liquid yielded a "snow" of solid .
Carbon dioxide in combination with nitrogen was known from earlier times as Blackdamp, stythe or choke damp. Along with the other types of damp it was encountered in mining operations and well sinking. Slow oxidation of coal and biological processes replaced the oxygen to create a suffocating mixture of nitrogen and carbon dioxide.
See also
*
*
* (from the atmosphere)
* (early work on and climate change)
*
* List of countries by carbon dioxide emissions
This is a list of sovereign states and territories by carbon dioxide emissions due to certain forms of human activity, based on thEDGAR databasecreated by European Commission and Netherlands Environmental Assessment Agency. The following ...
* List of least carbon efficient power stations
*
* NASA
The National Aeronautics and Space Administration (NASA ) is an independent agencies of the United States government, independent agency of the federal government of the United States, US federal government responsible for the United States ...
's
*
Notes
References
External links
Current global map of carbon dioxide concentration
* ttps://gml.noaa.gov/ccgg/trends/ Trends in Atmospheric Carbon Dioxide(NOAA)
The rediscovery of : History, What is Shecco?
- as refrigerant
A refrigerant is a working fluid used in the cooling, heating, or reverse cooling/heating cycles of air conditioning systems and heat pumps, where they undergo a repeated phase transition from a liquid to a gas and back again. Refrigerants are ...
{{DEFAULTSORT:Carbon Dioxide
Acid anhydrides
Acidic oxides
Coolants
Fire suppression agents
Greenhouse gases
Household chemicals
Inorganic solvents
Laser gain media
Nuclear reactor coolants
Oxocarbons
Propellants
Refrigerants
Gaseous signaling molecules
E-number additives
Triatomic molecules
 Carbon dioxide is colorless. At low concentrations, the gas is odorless; however, at sufficiently high concentrations, it has a sharp, acidic odor. At standard temperature and pressure, the density of carbon dioxide is around 1.98 kg/m3, about 1.53 times that of air.
Carbon dioxide has no liquid state at pressures below 0.51795(10) MPa (5.11177(99) atm). At a pressure of 1 atm (0.101325 MPa), the gas deposits directly to a solid at temperatures below 194.6855(30) K (−78.4645(30) °C) and the solid sublimes directly to a gas above this temperature. In its solid state, carbon dioxide is commonly called
Carbon dioxide is colorless. At low concentrations, the gas is odorless; however, at sufficiently high concentrations, it has a sharp, acidic odor. At standard temperature and pressure, the density of carbon dioxide is around 1.98 kg/m3, about 1.53 times that of air.
Carbon dioxide has no liquid state at pressures below 0.51795(10) MPa (5.11177(99) atm). At a pressure of 1 atm (0.101325 MPa), the gas deposits directly to a solid at temperatures below 194.6855(30) K (−78.4645(30) °C) and the solid sublimes directly to a gas above this temperature. In its solid state, carbon dioxide is commonly called  Plants can grow as much as 50% faster in concentrations of 1,000 ppm when compared with ambient conditions, though this assumes no change in climate and no limitation on other nutrients. Elevated levels cause increased growth reflected in the harvestable yield of crops, with wheat, rice and soybean all showing increases in yield of 12–14% under elevated in FACE experiments.
Increased atmospheric concentrations result in fewer stomata developing on plants which leads to reduced water usage and increased water-use efficiency. Studies using FACE have shown that enrichment leads to decreased concentrations of micronutrients in crop plants. This may have knock-on effects on other parts of
Plants can grow as much as 50% faster in concentrations of 1,000 ppm when compared with ambient conditions, though this assumes no change in climate and no limitation on other nutrients. Elevated levels cause increased growth reflected in the harvestable yield of crops, with wheat, rice and soybean all showing increases in yield of 12–14% under elevated in FACE experiments.
Increased atmospheric concentrations result in fewer stomata developing on plants which leads to reduced water usage and increased water-use efficiency. Studies using FACE have shown that enrichment leads to decreased concentrations of micronutrients in crop plants. This may have knock-on effects on other parts of  Adaptation to increased concentrations of occurs in humans, including modified breathing and kidney bicarbonate production, in order to balance the effects of blood acidification ( acidosis). Several studies suggested that 2.0 percent inspired concentrations could be used for closed air spaces (e.g. a
Adaptation to increased concentrations of occurs in humans, including modified breathing and kidney bicarbonate production, in order to balance the effects of blood acidification ( acidosis). Several studies suggested that 2.0 percent inspired concentrations could be used for closed air spaces (e.g. a  Poor ventilation is one of the main causes of excessive concentrations in closed spaces, leading to poor indoor air quality. Carbon dioxide differential above outdoor concentrations at steady state conditions (when the occupancy and ventilation system operation are sufficiently long that concentration has stabilized) are sometimes used to estimate ventilation rates per person. Higher concentrations are associated with occupant health, comfort and performance degradation.
Poor ventilation is one of the main causes of excessive concentrations in closed spaces, leading to poor indoor air quality. Carbon dioxide differential above outdoor concentrations at steady state conditions (when the occupancy and ventilation system operation are sufficiently long that concentration has stabilized) are sometimes used to estimate ventilation rates per person. Higher concentrations are associated with occupant health, comfort and performance degradation. 

 Around 230 Mt of are used each year, mostly in the fertiliser industry for urea production (130 million tonnes) and in the oil and gas industry for enhanced oil recovery (70 to 80 million tonnes). Other commercial applications include food and beverage production, metal fabrication, cooling, fire suppression and stimulating plant growth in greenhouses.
Technology exists to capture from industrial flue gas or from the air. Research is ongoing on ways to use captured in products and some of these processes have been deployed commercially. Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License However, the potential to use products is very small compared to the total volume of that could foreseeably be captured. Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License The vast majority of captured is considered a waste product and sequestered in underground geologic formations.Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License
Around 230 Mt of are used each year, mostly in the fertiliser industry for urea production (130 million tonnes) and in the oil and gas industry for enhanced oil recovery (70 to 80 million tonnes). Other commercial applications include food and beverage production, metal fabrication, cooling, fire suppression and stimulating plant growth in greenhouses.
Technology exists to capture from industrial flue gas or from the air. Research is ongoing on ways to use captured in products and some of these processes have been deployed commercially. Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License However, the potential to use products is very small compared to the total volume of that could foreseeably be captured. Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License The vast majority of captured is considered a waste product and sequestered in underground geologic formations.Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License
 Carbon dioxide is a
Carbon dioxide is a  Carbon dioxide in the form of
Carbon dioxide in the form of  Carbon dioxide can be used to extinguish flames by flooding the environment around the flame with the gas. It does not itself react to extinguish the flame, but starves the flame of oxygen by displacing it. Some fire extinguishers, especially those designed for electrical fires, contain liquid carbon dioxide under pressure. Carbon dioxide extinguishers work well on small flammable liquid and electrical fires, but not on ordinary combustible fires, because they do not cool the burning substances significantly, and when the carbon dioxide disperses, they can catch fire upon exposure to atmospheric oxygen. They are mainly used in server rooms.
Carbon dioxide has also been widely used as an extinguishing agent in fixed fire-protection systems for local application of specific hazards and total flooding of a protected space.
Carbon dioxide can be used to extinguish flames by flooding the environment around the flame with the gas. It does not itself react to extinguish the flame, but starves the flame of oxygen by displacing it. Some fire extinguishers, especially those designed for electrical fires, contain liquid carbon dioxide under pressure. Carbon dioxide extinguishers work well on small flammable liquid and electrical fires, but not on ordinary combustible fires, because they do not cool the burning substances significantly, and when the carbon dioxide disperses, they can catch fire upon exposure to atmospheric oxygen. They are mainly used in server rooms.
Carbon dioxide has also been widely used as an extinguishing agent in fixed fire-protection systems for local application of specific hazards and total flooding of a protected space.  Carbon dioxide is the lasing medium in a carbon-dioxide laser, which is one of the earliest type of lasers.
Carbon dioxide can be used as a means of controlling the pH of swimming pools, by continuously adding gas to the water, thus keeping the pH from rising. Among the advantages of this is the avoidance of handling (more hazardous) acids. Similarly, it is also used in the maintaining reef aquaria, where it is commonly used in calcium reactors to temporarily lower the pH of water being passed over
Carbon dioxide is the lasing medium in a carbon-dioxide laser, which is one of the earliest type of lasers.
Carbon dioxide can be used as a means of controlling the pH of swimming pools, by continuously adding gas to the water, thus keeping the pH from rising. Among the advantages of this is the avoidance of handling (more hazardous) acids. Similarly, it is also used in the maintaining reef aquaria, where it is commonly used in calcium reactors to temporarily lower the pH of water being passed over  Carbon dioxide was the first gas to be described as a discrete substance. In about 1640, the Flemish chemist
Carbon dioxide was the first gas to be described as a discrete substance. In about 1640, the Flemish chemist