|
Carbamino
Carbamino refers to an adduct generated by the addition of carbon dioxide to the free amino group of an amino acid or a protein, such as hemoglobin forming carbaminohemoglobin. Determining quantity of carboamino in products It is possible to determine how much carbamino is formed through the techniques of electron ionization and mass spectrometry. In determining the amount of product by mass spectrometry, a careful set of instructions are followed which allows for the carbamino adducts to be transferred to a vacuum for mass spectrometry. With the separation of the carbamino adducts in the ion sampling process, it should be that the pH does not change. Hence, mass spectrometry and electron ionization are a way to measure how much carbamino adduct there is in comparison to concentration of peptide in a solution. Formation of sugar-carbamino The sugar-carbamino is formed through a C-glycosidic linkage with the amino acid side chain via various linkers. The synthesis involves intro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbaminohemoglobin
Carbaminohemoglobin (carbaminohaemoglobin BrE) (CO2Hb, also known as carbhemoglobin and carbohemoglobin) is a Chemical compound, compound of hemoglobin and carbon dioxide, and is one of the forms in which carbon dioxide exists in the blood. In blood, 23% of carbon dioxide is carried this way, while 70% is converted into bicarbonate by carbonic anhydrase and then carried in plasma, and 7% carried as free CO2, dissolved in plasma. Structure The structure of carbaminohemoglobin can be described as the binding of carbon dioxide to the amino groups of the globin chains of hemoglobin. This occurs at the N-terminals of the globin chains and at the amino sidebranches of arginine and lysine residues. The process of carbon dioxide binding to hemoglobin is generally known as carbamino formation. This is the source from where the protein gets its name, as it is a combination of carbamino and hemoglobin. Function One of the primary functions of carbaminohemoglobin is to enable the trans ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hemoglobin
Hemoglobin (haemoglobin, Hb or Hgb) is a protein containing iron that facilitates the transportation of oxygen in red blood cells. Almost all vertebrates contain hemoglobin, with the sole exception of the fish family Channichthyidae. Hemoglobin in the blood carries oxygen from the respiratory organs (lungs or gills) to the other tissues of the body, where it releases the oxygen to enable aerobic respiration which powers an animal's metabolism. A healthy human has 12to 20grams of hemoglobin in every 100mL of blood. Hemoglobin is a metalloprotein, a chromoprotein, and a globulin. In mammals, hemoglobin makes up about 96% of a red blood cell's dry matter, dry weight (excluding water), and around 35% of the total weight (including water). Hemoglobin has an oxygen-binding capacity of 1.34mL of O2 per gram, which increases the total blood oxygen capacity seventy-fold compared to dissolved oxygen in blood plasma alone. The mammalian hemoglobin molecule can bind and transport up to four ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at normally-encountered concentrations it is odorless. As the source of carbon in the carbon cycle, atmospheric is the primary carbon source for life on Earth. In the air, carbon dioxide is transparent to visible light but absorbs infrared, infrared radiation, acting as a greenhouse gas. Carbon dioxide is soluble in water and is found in groundwater, lakes, ice caps, and seawater. It is a trace gas Carbon dioxide in Earth's atmosphere, in Earth's atmosphere at 421 parts per million (ppm), or about 0.042% (as of May 2022) having risen from pre-industrial levels of 280 ppm or about 0.028%. Burning fossil fuels is the main cause of these increased concentrations, which are the primary cause of climate change.IPCC (2022Summary for pol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adduct
In chemistry, an adduct (; alternatively, a contraction of "addition product") is a product of a direct addition of two or more distinct molecules, resulting in a single reaction product containing all atoms of all components. The resultant is considered a distinct molecular species. Examples include the addition of sodium bisulfite to an aldehyde to give a sulfonate. It can be considered as a single product resulting from the direct combination of different molecules which comprises all atoms of the reactant molecules. Adducts often form between Lewis acids and Lewis bases. A good example is the formation of adducts between the Lewis acid borane and the oxygen atom in the Lewis bases, tetrahydrofuran (THF): or diethyl ether: . Many Lewis acids and Lewis bases reacting in the gas phase or in non-aqueous solvents to form adducts have been examined in the ECW model. Trimethylborane, trimethyltin chloride and bis(hexafluoroacetylacetonato)copper(II) are examples of Lewi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of electrons. Amines can also exist as hetero cyclic compounds. Aniline is the simplest aromatic amine, consisting of a benzene ring bonded to an amino group. Amines are classified into three types: primary (1°), secondary (2°), and tertiary (3°) amines. Primary amines (1°) contain one alkyl or aryl substituent and have the general formula RNH2. Secondary amines (2°) have two alkyl or aryl groups attached to the nitrogen atom, with the general formula R2NH. Tertiary amines (3°) contain three substituent groups bonded to the nitrogen atom, and are represented by the formula R3N. The functional group present in primary amines is called the amino group. Classification of amines Amines can be classified according to the nature and number o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 appear in the genetic code of life. Amino acids can be classified according to the locations of the core structural functional groups ( alpha- , beta- , gamma- amino acids, etc.); other categories relate to polarity, ionization, and side-chain group type ( aliphatic, acyclic, aromatic, polar, etc.). In the form of proteins, amino-acid '' residues'' form the second-largest component (water being the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis. It is thought that they played a key role in enabling life on Earth and its emergence. Amino acids are formally named by the IUPAC- IUBMB Joint Commi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metabolic reactions, DNA replication, Cell signaling, responding to stimuli, providing Cytoskeleton, structure to cells and Fibrous protein, organisms, and Intracellular transport, transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the Nucleic acid sequence, nucleotide sequence of their genes, and which usually results in protein folding into a specific Protein structure, 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called pep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
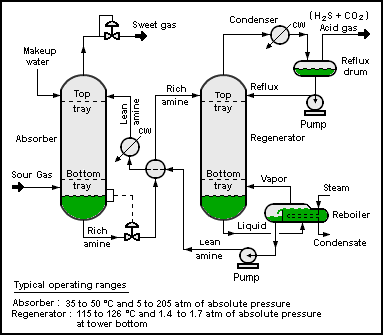
Amine Gas Treating
Amine gas treating, also known as amine scrubbing, gas sweetening and acid gas removal, refers to a group of processes that use aqueous solutions of various Amine#Aliphatic amines, alkylamines (commonly referred to simply as amines) to remove hydrogen sulfide (H2S) and carbon dioxide (CO2) from gases. It is a common unit process used in oil refinery, refineries, and is also used in petrochemical plants, natural gas processing, natural gas processing plants and other industries. Processes within oil refineries or chemical processing plants that remove Hydrogen Sulfide are referred to as "sweetening" processes because the odor of the processed products is improved by the absence of "sour" hydrogen sulfide. An alternative to the use of amines involves membrane technology. However, membrane separation is less attractive due to the relatively high capital and operating costs as well as other technical factors. Many different amines are used in gas treating: * Diethanolamine (DEA) * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ionic Liquids In Carbon Capture
The use of ionic liquids in carbon capture is a potential application of ionic liquids as absorbents for use in carbon capture and sequestration. Ionic liquids, which are salts that exist as liquids near room temperature, are polar, nonvolatile materials that have been considered for many applications. The urgency of climate change has spurred research into their use in energy-related applications such as carbon capture and storage. Carbon capture using absorption Ionic liquids as solvents Amines are the most prevalent absorbent in postcombustion carbon capture technology today. In particular, monoethanolamine (MEA) has been used in industrial scales in postcombustion carbon capture, as well as in other CO2 separations, such as "sweetening" of natural gas. However, amines are corrosive, degrade over time, and require large industrial facilities. Ionic liquids on the other hand, have low vapor pressures . This property results from their strong Coulombic attractive force. Va ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Functional Groups
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. This enables systematic prediction of chemical reactions and behavior of chemical compounds and the design of chemical synthesis. The Reactivity (chemistry), reactivity of a functional group can be modified by other functional groups nearby. Functional group interconversion can be used in retrosynthetic analysis to plan organic synthesis. A functional group is a group of atoms in a molecule with distinctive Chemical property, chemical properties, regardless of the other atoms in the molecule. The atoms in a functional group are linked to each other and to the rest of the molecule by covalent bonds. For repeating units of polymers, functional groups attach to their Chemical polarity, nonp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |