|
Unbibium
Unbibium, also known as element 122 or eka-thorium, is a hypothetical chemical element; it has placeholder symbol Ubb and atomic number 122. ''Unbibium'' and ''Ubb'' are the temporary Systematic element name, systematic IUPAC name and symbol respectively, which are used until the element is discovered, confirmed, and a permanent name is decided upon. In the periodic table of the elements, it is expected to follow unbiunium as the second element of the superactinides and the fourth element of the 8th Period (periodic table), period. Similarly to unbiunium, it is expected to fall within the range of the island of stability, potentially conferring additional stability on some isotopes, especially 306Ubb which is expected to have a Magic number (physics), magic number of neutrons (184). Despite several attempts, unbibium has not yet been synthesized, nor have any naturally occurring isotopes been found to exist. There are currently no plans to attempt to synthesize unbibium. In 2008, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Superactinides
An extended periodic table theorizes about chemical elements beyond those currently known and proven. The element with the highest atomic number known is oganesson (''Z'' = 118), which completes the seventh period (row) in the periodic table. All elements in the eighth period and beyond thus remain purely hypothetical. Elements beyond 118 will be placed in additional periods when discovered, laid out (as with the existing periods) to illustrate periodically recurring trends in the properties of the elements. Any additional periods are expected to contain more elements than the seventh period, as they are calculated to have an additional so-called ''g-block'', containing at least 18 elements with partially filled g- orbitals in each period. An ''eight-period table'' containing this block was suggested by Glenn T. Seaborg in 1969. The first element of the g-block may have atomic number 121, and thus would have the systematic name unbiunium. Despite many searches, no e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amnon Marinov
Amnon Marinov (; 1930 – 2011) was an Israeli physicist. He undertook research into nuclear structures, nuclear reactions, superheavy elements and long-lived nuclear isomers. Claimed discovery of unbibium On April 24, 2008, a group led by Marinov at Hebrew University of Jerusalem claimed to have found single atoms of unbibium-292 in natural thorium deposits at an abundance of 10−11 to 10−12 relative to thorium. This was the first time in sixty-nine years that a new element had been claimed to be discovered in nature, after Marguerite Perey's 1939 discovery of francium. The claim of Marinov ''et al.'' was criticized by a part of the scientific community, and Marinov said he submitted the article to the journals ''Nature'' and ''Nature Physics'' but both turned it down without sending it for peer review. The unbibium-292 atoms were claimed to be superdeformed or hyperdeformed nuclear isomers, with a half-life of at least 108 years. A criticism of the technique, previously used ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Unbiunium
Unbiunium, also known as eka-actinium or element 121, is a hypothetical chemical element; it has symbol Ubu and atomic number 121. ''Unbiunium'' and ''Ubu'' are the temporary systematic IUPAC name and symbol respectively, which are used until the element is discovered, confirmed, and a permanent name is decided upon. In the periodic table of the elements, it is expected to be the first of the superactinides, and the third element in the eighth period. It has attracted attention because of some predictions that it may be in the island of stability. It is also likely to be the first of a new g-block of elements. Unbiunium has not yet been synthesized. It is expected to be one of the last few reachable elements with current technology; the limit could be anywhere between element 120 and 124. It will also likely be far more difficult to synthesize than the elements known so far up to 118, and still more difficult than elements 119 and 120. The teams at RIKEN in Japan and at the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Systematic Element Name
A systematic element name is the temporary name assigned to an unknown or recently synthesized chemical element. A systematic symbol is also derived from this name. In chemistry, a transuranic element receives a permanent name and symbol only after its synthesis has been confirmed. In some cases, such as the Transfermium Wars, controversies over the formal name and symbol have been protracted and highly political. In order to discuss such elements without ambiguity, the International Union of Pure and Applied Chemistry (IUPAC) uses a set of rules, adopted in 1978, to assign a temporary systematic name and symbol to each such element. This approach to naming originated in the successful development of regular rules for the naming of organic compounds. IUPAC rules The temporary names derive systematically from the element's atomic number, and apply only to 101 ≤ ''Z'' ≤ 999. Each digit is translated into a "numerical root" according to the table. The root ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
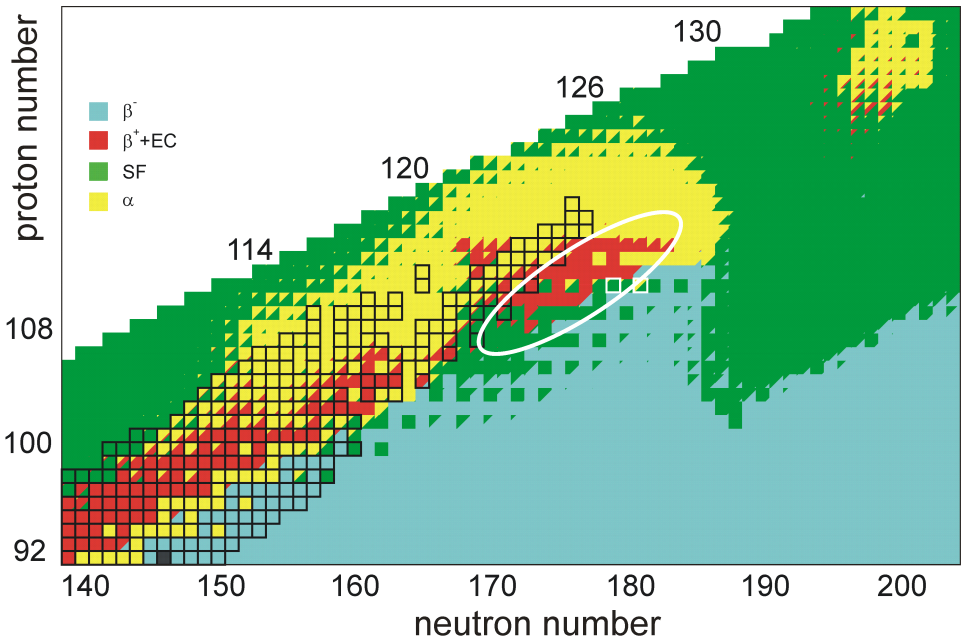
Island Of Stability
In nuclear physics, the island of stability is a predicted set of isotopes of superheavy elements that may have considerably longer half-lives than known isotopes of these elements. It is predicted to appear as an "island" in the chart of nuclides, separated from known stable nuclide, stable and long-lived primordial radionuclides. Its theoretical existence is attributed to stabilizing effects of predicted "Magic number (physics), magic numbers" of protons and neutrons in the superheavy mass region. Several predictions have been made regarding the exact location of the island of stability, though it is generally thought to center near copernicium and flerovium isotopes in the vicinity of the predicted closed neutron nuclear shell model, shell at ''N'' = 184. These models strongly suggest that the closed shell will confer further stability towards nuclear fission, fission and alpha decay. While these effects are expected to be greatest near atomic number ''Z'' =&nb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flerovium
Flerovium is a synthetic chemical element; it has symbol Fl and atomic number 114. It is an extremely radioactive, superheavy element, named after the Flerov Laboratory of Nuclear Reactions of the Joint Institute for Nuclear Research in Dubna, Russia, where the element was discovered in 1999. The lab's name, in turn, honours Russian physicist Georgy Flyorov ( in Cyrillic, hence the transliteration of " yo" to "e"). IUPAC adopted the name on 30 May 2012. The name and symbol had previously been proposed for element 102 (nobelium) but were not accepted by IUPAC at that time. It is a transactinide in the p-block of the periodic table. It is in period 7 and is the heaviest known member of the carbon group. Initial chemical studies in 2007–2008 indicated that flerovium was unexpectedly volatile for a group 14 element. More recent results show that flerovium's reaction with gold is similar to that of copernicium, showing it is very volatile and may even be gaseous at stan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Element
A chemical element is a chemical substance whose atoms all have the same number of protons. The number of protons is called the atomic number of that element. For example, oxygen has an atomic number of 8: each oxygen atom has 8 protons in its atomic nucleus, nucleus. Atoms of the same element can have different numbers of neutrons in their nuclei, known as isotopes of the element. Two or more atoms can combine to form molecules. Some elements form Homonuclear molecule, molecules of atoms of said element only: e.g. atoms of hydrogen (H) form Diatomic molecule, diatomic molecules (H). Chemical compounds are substances made of atoms of different elements; they can have molecular or non-molecular structure. Mixtures are materials containing different chemical substances; that means (in case of molecular substances) that they contain different types of molecules. Atoms of one element can be transformed into atoms of a different element in nuclear reactions, which change an atom's at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hebrew University Of Jerusalem
The Hebrew University of Jerusalem (HUJI; ) is an Israeli public university, public research university based in Jerusalem. Co-founded by Albert Einstein and Chaim Weizmann in July 1918, the public university officially opened on 1 April 1925. It is the second-oldest Israeli university, having been founded 30 years before the Israeli Declaration of Independence, establishment of the State of Israel but six years after the older Technion university. The HUJI has three campuses in Jerusalem: one in Rehovot, one in Rishon LeZion and one in Eilat. Until 2023, the world's largest library for Jewish studies—the National Library of Israel—was located on its Edmond Safra, Edmond J. Safra campus in the Givat Ram neighbourhood of Jerusalem. The university has five affiliated teaching hospitals (including the Hadassah Medical Center), seven faculties, more than 100 research centers, and 315 academic departments. , one-third of all the doctoral candidates in Israel were studying at the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flerov Laboratory Of Nuclear Reactions
The Joint Institute for Nuclear Research (JINR, ), in Dubna, Moscow Oblast (110 km north of Moscow), Russia, is an international research center for nuclear sciences, with 5,500 staff members including 1,200 researchers holding over 1,000 Ph.D.s from eighteen countries. Most scientists are scientists of the Russian Federation. The institute has seven laboratories, each with its own specialisation: theoretical physics, high energy physics (particle physics), heavy ion physics, condensed matter physics, nuclear reactions, neutron physics, and information technology. The institute has a division to study radiation and radiobiological research and other ad hoc experimental physics experiments. Principal research instruments include a nuclotron superconductive particle accelerator (particle energy: 7 GeV), three isochronous cyclotrons (120, 145, 650 MeV), a phasitron (680 MeV) and a synchrophasotron (4 GeV). The site has a neutron fast-pulse reactor (1,500MW pulse) with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Shell Model
In nuclear physics, atomic physics, and nuclear chemistry, the nuclear shell model utilizes the Pauli exclusion principle to model the structure of atomic nuclei in terms of energy levels. The first shell model was proposed by Dmitri Ivanenko (together with E. Gapon) in 1932. The model was developed in 1949 following independent work by several physicists, most notably Maria Goeppert Mayer and J. Hans D. Jensen, who received the 1963 Nobel Prize in Physics for their contributions to this model, and Eugene Wigner, who received the Nobel Prize alongside them for his earlier groundlaying work on the atomic nuclei. The nuclear shell model is partly analogous to the atomic shell model, which describes the arrangement of electrons in an atom, in that a filled shell results in better stability. When adding nucleons (protons and neutrons) to a nucleus, there are certain points where the binding energy of the next nucleon is significantly less than the last one. This observation th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Astatine
Astatine is a chemical element; it has Symbol (chemistry), symbol At and atomic number 85. It is the abundance of elements in Earth's crust, rarest naturally occurring element in the Earth's crust, occurring only as the Decay chain, decay product of various heavier elements. All of astatine's isotopes are short-lived; the most stable is astatine-210, with a half-life of 8.1 hours. Consequently, a solid sample of the element has never been seen, because any macroscopic specimen would be immediately vaporized by the heat of its radioactivity. The bulk properties of astatine are not known with certainty. Many of them have been estimated from its position on the periodic table as a heavier analog of fluorine, chlorine, bromine, and iodine, the four stable halogens. However, astatine also falls roughly along the dividing line between metals and nonmetals, and some metallic behavior has also been observed and predicted for it. Astatine is likely to have a dark or lustrous appearanc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Marguerite Perey
Marguerite Catherine Perey (19 October 1909 – 13 May 1975) was a French physicist and a student of Marie Curie. In 1939, Perey discovered the element francium by purifying samples of lanthanum that contained actinium. In 1962, she was the first woman to be elected to the French Académie des Sciences, an honor denied to her mentor Curie. Perey died of cancer in 1975. Early life Perey was born in 1909 in Villemomble, France, just outside Paris where the Curie's Radium Institute was located. Although she hoped to study medicine, the death of her father left the family in financial difficulties. Perey earned a chemistry diploma from Paris' Technical School of Women's Education in 1929; while not a "degree", it did qualify her to work as a chemistry technician. In 1929 at the age of 19, Perey interviewed for a role as a personal assistant (technician) to Marie Curie at Curie's Radium Institute in Paris, France, and was hired. Marie Curie took on a mentoring role to Perey, tak ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |