chemical element on:
[Wikipedia]
[Google]
[Amazon]
 A chemical element is a species of atoms that have a given number of
A chemical element is a species of atoms that have a given number of
 Only about 4% of the total mass of the universe is made of atoms or ions, and thus represented by chemical elements. This fraction is about 15% of the total matter, with the remainder of the matter (85%) being dark matter. The nature of dark matter is unknown, but it is not composed of atoms of chemical elements because it contains no protons, neutrons, or electrons. (The remaining non-matter part of the mass of the universe is composed of the even less well understood dark energy).
The 94 naturally occurring chemical elements were produced by at least four classes of astrophysical process. Most of the hydrogen, helium and a very small quantity of lithium were produced in the first few minutes of the
Only about 4% of the total mass of the universe is made of atoms or ions, and thus represented by chemical elements. This fraction is about 15% of the total matter, with the remainder of the matter (85%) being dark matter. The nature of dark matter is unknown, but it is not composed of atoms of chemical elements because it contains no protons, neutrons, or electrons. (The remaining non-matter part of the mass of the universe is composed of the even less well understood dark energy).
The 94 naturally occurring chemical elements were produced by at least four classes of astrophysical process. Most of the hydrogen, helium and a very small quantity of lithium were produced in the first few minutes of the  On Earth (and elsewhere), trace amounts of various elements continue to be produced from other elements as products of
On Earth (and elsewhere), trace amounts of various elements continue to be produced from other elements as products of

 From Boyle until the early 20th century, an element was defined as a pure substance that could not be decomposed into any simpler substance. Put another way, a chemical element cannot be transformed into other chemical elements by chemical processes. Elements during this time were generally distinguished by their atomic weights, a property measurable with fair accuracy by available analytical techniques.
From Boyle until the early 20th century, an element was defined as a pure substance that could not be decomposed into any simpler substance. Put another way, a chemical element cannot be transformed into other chemical elements by chemical processes. Elements during this time were generally distinguished by their atomic weights, a property measurable with fair accuracy by available analytical techniques.
Videos for each element
by the University of Nottingham
"Chemical Elements"
''In Our Time'', BBC Radio 4 discussion with Paul Strathern, Mary Archer and John Murrell (25 May 2000) {{Authority control Chemistry
 A chemical element is a species of atoms that have a given number of
A chemical element is a species of atoms that have a given number of proton
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ...
s in their nuclei, including the pure substance
Substance may refer to:
* Matter, anything that has mass and takes up space
Chemistry
* Chemical substance, a material with a definite chemical composition
* Drug substance
** Substance abuse, drug-related healthcare and social policy diagnosis ...
consisting only of that species. Unlike chemical compounds, chemical elements cannot be broken down into simpler substances by any chemical reaction. The number of protons in the nucleus is the defining property of an element, and is referred to as its atomic number (represented by the symbol ''Z'') – all atoms with the same atomic number are atoms of the same element. Almost all of the baryonic
In particle physics, a baryon is a type of composite particle, composite subatomic particle which contains an odd number of valence quarks (at least 3). Baryons belong to the hadron list of particles, family of particles; hadrons are composed o ...
matter of the universe is composed of chemical elements (among rare exceptions are neutron stars). When different elements undergo chemical reactions, atoms are rearranged into new compounds held together by chemical bonds. Only a minority of elements, such as silver and gold, are found uncombined as relatively pure native element minerals. Nearly all other naturally occurring elements occur in the Earth as compounds or mixture
In chemistry, a mixture is a material made up of two or more different chemical substances which are not chemically bonded. A mixture is the physical combination of two or more substances in which the identities are retained and are mixed in the ...
s. Air is primarily a mixture of the elements nitrogen, oxygen, and argon, though it does contain compounds including carbon dioxide and water.
The history of the discovery and use of the elements began with primitive
Primitive may refer to:
Mathematics
* Primitive element (field theory)
* Primitive element (finite field)
* Primitive cell (crystallography)
* Primitive notion, axiomatic systems
* Primitive polynomial (disambiguation), one of two concepts
* Pr ...
human societies
A society is a group of individuals involved in persistent social interaction, or a large social group sharing the same spatial or social territory, typically subject to the same political authority and dominant cultural expectations. Societi ...
that discovered native minerals like carbon, sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula ...
, copper and gold (though the concept of a chemical element was not yet understood). Attempts to classify materials such as these resulted in the concepts of classical element
Classical elements typically refer to earth, water, air, fire, and (later) aether which were proposed to explain the nature and complexity of all matter in terms of simpler substances. Ancient cultures in Greece, Tibet, and India had simil ...
s, alchemy, and various similar theories throughout human history. Much of the modern understanding of elements developed from the work of Dmitri Mendeleev
Dmitri Ivanovich Mendeleev (sometimes transliterated as Mendeleyev or Mendeleef) ( ; russian: links=no, Дмитрий Иванович Менделеев, tr. , ; 8 February Old_Style_and_New_Style_dates">O.S._27_January.html" ;"title="O ...
, a Russian chemist who published the first recognizable periodic table
The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of ch ...
in 1869. This table organizes the elements by increasing atomic number into rows (" periods") in which the columns (" groups") share recurring ("periodic") physical
Physical may refer to:
*Physical examination
In a physical examination, medical examination, or clinical examination, a medical practitioner examines a patient for any possible medical signs or symptoms of a medical condition. It generally co ...
and chemical properties. The periodic table summarizes various properties of the elements, allowing chemists to derive relationships between them and to make predictions about compounds and potential new ones.
By November 2016, the International Union of Pure and Applied Chemistry had recognized a total of 118 elements. The first 94 occur naturally on Earth, and the remaining 24 are synthetic elements produced in nuclear reactions. Save for unstable radioactive elements (radionuclide
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess nuclear energy, making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transfer ...
s) which decay quickly, nearly all of the elements are available industrially in varying amounts. The discovery and synthesis of further new elements is an ongoing area of scientific study.
Description
The lightest chemical elements are hydrogen and helium, both created by Big Bang nucleosynthesis during the first 20 minutes of the universe in a ratio of around 3:1 by mass (or 12:1 by number of atoms), along with tiny traces of the next two elements, lithium and beryllium. Almost all other elements found in nature were made by various natural methods of nucleosynthesis. On Earth, small amounts of new atoms are naturally produced in nucleogenic reactions, or in cosmogenic processes, such as cosmic ray spallation. New atoms are also naturally produced on Earth as radiogenic daughter isotopes of ongoingradioactive decay
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is consid ...
processes such as alpha decay, beta decay, spontaneous fission
Spontaneous fission (SF) is a form of radioactive decay that is found only in very heavy chemical elements. The nuclear binding energy of the elements reaches its maximum at an atomic mass number of about 56 (e.g., iron-56); spontaneous breakdo ...
, cluster decay
Cluster decay, also named heavy particle radioactivity or heavy ion radioactivity, is a rare type of nuclear decay in which an atomic nucleus emits a small "cluster" of neutrons and protons, more than in an alpha particle, but less than a typic ...
, and other rarer modes of decay.
Of the 94 naturally occurring elements, those with atomic numbers 1 through 82 each have at least one stable isotope (except for technetium, element 43 and promethium
Promethium is a chemical element with the symbol Pm and atomic number 61. All of its isotopes are radioactive; it is extremely rare, with only about 500–600 grams naturally occurring in Earth's crust at any given time. Promethium is one of onl ...
, element 61, which have no stable isotopes). Isotopes considered stable are those for which no radioactive decay has yet been observed. Elements with atomic numbers 83 through 94 are unstable to the point that radioactive decay of all isotopes can be detected. Some of these elements, notably bismuth (atomic number 83), thorium (atomic number 90), and uranium (atomic number 92), have one or more isotopes with half-lives long enough to survive as remnants of the explosive stellar nucleosynthesis
Stellar nucleosynthesis is the creation (nucleosynthesis) of chemical elements by nuclear fusion reactions within stars. Stellar nucleosynthesis has occurred since the original creation of hydrogen, helium and lithium during the Big Bang. As a ...
that produced the heavy metals
upright=1.2, Crystals of osmium, a heavy metal nearly twice as dense as lead">lead.html" ;"title="osmium, a heavy metal nearly twice as dense as lead">osmium, a heavy metal nearly twice as dense as lead
Heavy metals are generally defined as ...
before the formation of our Solar System. At over 1.9 years, over a billion times longer than the current estimated age of the universe, bismuth-209 (atomic number 83) has the longest known alpha decay half-life of any naturally occurring element, and is almost always considered on par with the 80 stable elements. The very heaviest elements (those beyond plutonium, element 94) undergo radioactive decay with half-lives so short that they are not found in nature and must be synthesized.
There are now 118 known elements. In this context, "known" means observed well enough, even from just a few decay products, to have been differentiated from other elements. Most recently, the synthesis of element 118 (since named oganesson) was reported in October 2006, and the synthesis of element 117 ( tennessine) was reported in April 2010. Of these 118 elements, 94 occur naturally on Earth. Six of these occur in extreme trace quantities: technetium, atomic number 43; promethium
Promethium is a chemical element with the symbol Pm and atomic number 61. All of its isotopes are radioactive; it is extremely rare, with only about 500–600 grams naturally occurring in Earth's crust at any given time. Promethium is one of onl ...
, number 61; astatine, number 85; francium
Francium is a chemical element with the symbol Fr and atomic number 87. It is extremely radioactive; its most stable isotope, francium-223 (originally called actinium K after the natural decay chain it appears in), has a half-life of only 22&nb ...
, number 87; neptunium, number 93; and plutonium, number 94. These 94 elements have been detected in the universe at large, in the spectra of stars and also supernovae, where short-lived radioactive elements are newly being made. The first 94 elements have been detected directly on Earth as primordial nuclide
In geochemistry, geophysics and nuclear physics, primordial nuclides, also known as primordial isotopes, are nuclides found on Earth that have existed in their current form since before Earth was formed. Primordial nuclides were present in the ...
s present from the formation of the Solar System, or as naturally occurring fission or transmutation products of uranium and thorium.
The remaining 24 heavier elements, not found today either on Earth or in astronomical spectra, have been produced artificially: these are all radioactive, with very short half-lives; if any atoms of these elements were present at the formation of Earth, they are extremely likely, to the point of certainty, to have already decayed, and if present in novae have been in quantities too small to have been noted. Technetium was the first purportedly non-naturally occurring element synthesized, in 1937, although trace amounts of technetium have since been found in nature (and also the element may have been discovered naturally in 1925). This pattern of artificial production and later natural discovery has been repeated with several other radioactive naturally occurring rare elements.
List of the elements are available by name, atomic number, density, melting point, boiling point and by symbol, as well as ionization energies of the elements. The nuclides of stable and radioactive elements are also available as a list of nuclides, sorted by length of half-life for those that are unstable. One of the most convenient, and certainly the most traditional presentation of the elements, is in the form of the periodic table
The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of ch ...
, which groups together elements with similar chemical properties (and usually also similar electronic structures).
Atomic number
The atomic number of an element is equal to the number of protons in each atom, and defines the element. For example, all carbon atoms contain 6 protons in their atomic nucleus; so the atomic number of carbon is 6. Carbon atoms may have different numbers of neutrons; atoms of the same element having different numbers of neutrons are known as isotopes of the element. The number of protons in the atomic nucleus also determines its electric charge, which in turn determines the number of electrons of the atom in its non-ionized state. The electrons are placed into atomic orbitals that determine the atom's various chemical properties. The number of neutrons in a nucleus usually has very little effect on an element's chemical properties (except in the case of hydrogen and deuterium). Thus, all carbon isotopes have nearly identical chemical properties because they all have six protons and six electrons, even though carbon atoms may, for example, have 6 or 8 neutrons. That is why the atomic number, rather than mass number or atomic weight, is considered the identifying characteristic of a chemical element. The symbol for atomic number is ''Z''.Isotopes
Isotopes are atoms of the same element (that is, with the same number ofproton
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ...
s in their atomic nucleus), but having ''different'' numbers of neutrons. Thus, for example, there are three main isotopes of carbon. All carbon atoms have 6 protons in the nucleus, but they can have either 6, 7, or 8 neutrons. Since the mass numbers of these are 12, 13 and 14 respectively, the three isotopes of carbon are known as carbon-12
Carbon-12 (12C) is the most abundant of the two stable isotopes of carbon (carbon-13 being the other), amounting to 98.93% of element carbon on Earth; its abundance is due to the triple-alpha process by which it is created in stars. Carbon-12 i ...
, carbon-13, and carbon-14
Carbon-14, C-14, or radiocarbon, is a radioactive isotope of carbon with an atomic nucleus containing 6 protons and 8 neutrons. Its presence in organic materials is the basis of the radiocarbon dating method pioneered by Willard Libby and coll ...
, often abbreviated to 12C, 13C, and 14C. Carbon in everyday life and in chemistry is a mixture
In chemistry, a mixture is a material made up of two or more different chemical substances which are not chemically bonded. A mixture is the physical combination of two or more substances in which the identities are retained and are mixed in the ...
of 12C (about 98.9%), 13C (about 1.1%) and about 1 atom per trillion of 14C.
Most (66 of 94) naturally occurring elements have more than one stable isotope. Except for the isotopes of hydrogen (which differ greatly from each other in relative mass—enough to cause chemical effects), the isotopes of a given element are chemically nearly indistinguishable.
All of the elements have some isotopes that are radioactive ( radioisotopes), although not all of these radioisotopes occur naturally. The radioisotopes typically decay into other elements upon radiating an alpha
Alpha (uppercase , lowercase ; grc, ἄλφα, ''álpha'', or ell, άλφα, álfa) is the first letter of the Greek alphabet. In the system of Greek numerals, it has a value of one. Alpha is derived from the Phoenician letter aleph , whic ...
or beta particle
A beta particle, also called beta ray or beta radiation (symbol β), is a high-energy, high-speed electron or positron emitted by the radioactive decay of an atomic nucleus during the process of beta decay. There are two forms of beta decay, β� ...
. If an element has isotopes that are not radioactive, these are termed "stable" isotopes. All of the known stable isotopes occur naturally (see primordial isotope). The many radioisotopes that are not found in nature have been characterized after being artificially made. Certain elements have no stable isotopes and are composed ''only'' of radioactive isotopes: specifically the elements without any stable isotopes are technetium (atomic number 43), promethium (atomic number 61), and all observed elements with atomic numbers greater than 82.
Of the 80 elements with at least one stable isotope, 26 have only one single stable isotope. The mean number of stable isotopes for the 80 stable elements is 3.1 stable isotopes per element. The largest number of stable isotopes that occur for a single element is 10 (for tin, element 50).
Isotopic mass and atomic mass
The mass number of an element, ''A'', is the number of nucleons (protons and neutrons) in the atomic nucleus. Different isotopes of a given element are distinguished by their mass numbers, which are conventionally written as a superscript on the left hand side of the atomic symbol (e.g. 238U). The mass number is always a whole number and has units of "nucleons". For example, magnesium-24 (24 is the mass number) is an atom with 24 nucleons (12 protons and 12 neutrons). Whereas the mass number simply counts the total number of neutrons and protons and is thus a natural (or whole) number, the atomic mass of a single atom is a real number giving the mass of a particular isotope (or "nuclide") of the element, expressed in atomic mass units (symbol: u). In general, the mass number of a given nuclide differs in value slightly from its atomic mass, since the mass of each proton and neutron is not exactly 1 u; since the electrons contribute a lesser share to the atomic mass as neutron number exceeds proton number; and (finally) because of thenuclear binding energy
Nuclear binding energy in experimental physics is the minimum energy that is required to disassemble the atomic nucleus, nucleus of an atom into its constituent protons and neutrons, known collectively as nucleons. The binding energy for stable n ...
. For example, the atomic mass of chlorine-35 to five significant digits is 34.969 u and that of chlorine-37 is 36.966 u. However, the atomic mass in u of each isotope is quite close to its simple mass number (always within 1%). The only isotope whose atomic mass is exactly a natural number is 12C, which by definition has a mass of exactly 12 because u is defined as 1/12 of the mass of a free neutral carbon-12 atom in the ground state.
The standard atomic weight
The standard atomic weight of a chemical element (symbol ''A''r°(E) for element "E") is the weighted arithmetic mean of the relative isotopic masses of all isotopes of that element weighted by each isotope's abundance on Earth. For example, is ...
(commonly called "atomic weight") of an element is the ''average'' of the atomic masses of all the chemical element's isotopes as found in a particular environment, weighted by isotopic abundance, relative to the atomic mass unit. This number may be a fraction that is ''not'' close to a whole number. For example, the relative atomic mass of chlorine is 35.453 u, which differs greatly from a whole number as it is an average of about 76% chlorine-35 and 24% chlorine-37. Whenever a relative atomic mass value differs by more than 1% from a whole number, it is due to this averaging effect, as significant amounts of more than one isotope are naturally present in a sample of that element.
Chemically pure and isotopically pure
Chemists and nuclear scientists have different definitions of a ''pure element''. In chemistry, a pure element means a substance whose atoms all (or in practice almost all) have the same atomic number, or number ofproton
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ...
s. Nuclear scientists, however, define a pure element as one that consists of only one stable isotope.
For example, a copper wire is 99.99% chemically pure if 99.99% of its atoms are copper, with 29 protons each. However it is not isotopically pure since ordinary copper consists of two stable isotopes, 69% 63Cu and 31% 65Cu, with different numbers of neutrons. However, a pure gold ingot would be both chemically and isotopically pure, since ordinary gold consists only of one isotope, 197Au.
Allotropes
Atoms of chemically pure elements may bond to each other chemically in more than one way, allowing the pure element to exist in multiple chemical structures ( spatial arrangements of atoms), known as allotropes, which differ in their properties. For example, carbon can be found as diamond, which has a tetrahedral structure around each carbon atom; graphite, which has layers of carbon atoms with a hexagonal structure stacked on top of each other; graphene, which is a single layer of graphite that is very strong; fullerenes, which have nearly spherical shapes; andcarbon nanotube
A scanning tunneling microscopy image of a single-walled carbon nanotube
Rotating single-walled zigzag carbon nanotube
A carbon nanotube (CNT) is a tube made of carbon with diameters typically measured in nanometers.
''Single-wall carbon na ...
s, which are tubes with a hexagonal structure (even these may differ from each other in electrical properties). The ability of an element to exist in one of many structural forms is known as 'allotropy'.
The reference state of an element is defined by convention, usually as the thermodynamically most stable allotrope and physical state at a pressure of 1 bar
Bar or BAR may refer to:
Food and drink
* Bar (establishment), selling alcoholic beverages
* Candy bar
* Chocolate bar
Science and technology
* Bar (river morphology), a deposit of sediment
* Bar (tropical cyclone), a layer of cloud
* Bar (u ...
and a given temperature (typically at 298.15K). However, for phosphorus, the reference state is white phosphorus even though it is not the most stable allotrope. In thermochemistry, an element is defined to have an enthalpy of formation of zero in its reference state. For example, the reference state for carbon is graphite, because the structure of graphite is more stable than that of the other allotropes.
Properties
Several kinds of descriptive categorizations can be applied broadly to the elements, including consideration of their general physical and chemical properties, their states of matter under familiar conditions, their melting and boiling points, their densities, their crystal structures as solids, and their origins.General properties
Several terms are commonly used to characterize the general physical and chemical properties of the chemical elements. A first distinction is between metals, which readily conduct electricity,nonmetal
In chemistry, a nonmetal is a chemical element that generally lacks a predominance of metallic properties; they range from colorless gases (like hydrogen) to shiny solids (like carbon, as graphite). The electrons in nonmetals behave differentl ...
s, which do not, and a small group, (the ''metalloid
A metalloid is a type of chemical element which has a preponderance of material property, properties in between, or that are a mixture of, those of metals and nonmetals. There is no standard definition of a metalloid and no complete agreement on ...
s''), having intermediate properties and often behaving as semiconductors.
A more refined classification is often shown in colored presentations of the periodic table. This system restricts the terms "metal" and "nonmetal" to only certain of the more broadly defined metals and nonmetals, adding additional terms for certain sets of the more broadly viewed metals and nonmetals. The version of this classification used in the periodic tables presented here includes: actinides, alkali metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names ...
s, alkaline earth metals, halogen
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this group is ...
s, lanthanide
The lanthanide () or lanthanoid () series of chemical elements comprises the 15 metallic chemical elements with atomic numbers 57–71, from lanthanum through lutetium. These elements, along with the chemically similar elements scandium and yttr ...
s, transition metals, post-transition metals, metalloid
A metalloid is a type of chemical element which has a preponderance of material property, properties in between, or that are a mixture of, those of metals and nonmetals. There is no standard definition of a metalloid and no complete agreement on ...
s, reactive nonmetal
In chemistry, a nonmetal is a chemical element that generally lacks a predominance of metallic properties; they range from colorless gases (like hydrogen) to shiny solids (like carbon, as graphite). The electrons in nonmetals behave different ...
s, and noble gases. In this system, the alkali metals, alkaline earth metals, and transition metals, as well as the lanthanides and the actinides, are special groups of the metals viewed in a broader sense. Similarly, the reactive nonmetals and the noble gases are nonmetals viewed in the broader sense. In some presentations, the halogens are not distinguished, with astatine identified as a metalloid and the others identified as nonmetals.
States of matter
Another commonly used basic distinction among the elements is their state of matter (phase), whether solid,liquid
A liquid is a nearly incompressible fluid that conforms to the shape of its container but retains a (nearly) constant volume independent of pressure. As such, it is one of the four fundamental states of matter (the others being solid, gas, a ...
, or gas, at a selected standard temperature and pressure
Standard temperature and pressure (STP) are standard sets of conditions for experimental measurements to be established to allow comparisons to be made between different sets of data. The most used standards are those of the International Union o ...
(STP). Most of the elements are solids at conventional temperatures and atmospheric pressure, while several are gases. Only bromine and mercury
Mercury commonly refers to:
* Mercury (planet), the nearest planet to the Sun
* Mercury (element), a metallic chemical element with the symbol Hg
* Mercury (mythology), a Roman god
Mercury or The Mercury may also refer to:
Companies
* Merc ...
are liquids at 0 degrees Celsius (32 degrees Fahrenheit) and normal atmospheric pressure; caesium
Caesium (IUPAC spelling) (or cesium in American English) is a chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-golden alkali metal with a melting point of , which makes it one of only five elemental metals that a ...
and gallium
Gallium is a chemical element with the symbol Ga and atomic number 31. Discovered by French chemist Paul-Émile Lecoq de Boisbaudran in 1875, Gallium is in group 13 of the periodic table and is similar to the other metals of the group (aluminiu ...
are solids at that temperature, but melt at 28.4 °C (83.2 °F) and 29.8 °C (85.6 °F), respectively.
Melting and boiling points
Melting andboiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding envir ...
s, typically expressed in degrees Celsius at a pressure of one atmosphere, are commonly used in characterizing the various elements. While known for most elements, either or both of these measurements is still undetermined for some of the radioactive elements available in only tiny quantities. Since helium remains a liquid even at absolute zero
Absolute zero is the lowest limit of the thermodynamic temperature scale, a state at which the enthalpy and entropy of a cooled ideal gas reach their minimum value, taken as zero kelvin. The fundamental particles of nature have minimum vibration ...
at atmospheric pressure, it has only a boiling point, and not a melting point, in conventional presentations.
Densities
The density at selected standard temperature and pressure ( STP) is frequently used in characterizing the elements. Density is often expressed in grams per cubic centimeter (g/cm3). Since several elements are gases at commonly encountered temperatures, their densities are usually stated for their gaseous forms; when liquefied or solidified, the gaseous elements have densities similar to those of the other elements. When an element has allotropes with different densities, one representative allotrope is typically selected in summary presentations, while densities for each allotrope can be stated where more detail is provided. For example, the three familiarallotropes of carbon
Carbon is capable of forming many allotropy, allotropes (structurally different forms of the same element) due to its Valence (chemistry), valency. Well-known forms of carbon include diamond and graphite. In recent decades, many more allotrope ...
( amorphous carbon, graphite, and diamond) have densities of 1.8–2.1, 2.267, and 3.515 g/cm3, respectively.
Crystal structures
The elements studied to date as solid samples have eight kinds of crystal structures:cubic
Cubic may refer to:
Science and mathematics
* Cube (algebra), "cubic" measurement
* Cube, a three-dimensional solid object bounded by six square faces, facets or sides, with three meeting at each vertex
** Cubic crystal system, a crystal system w ...
, body-centered cubic, face-centered cubic, hexagonal, monoclinic, orthorhombic
In crystallography, the orthorhombic crystal system is one of the 7 crystal systems. Orthorhombic lattices result from stretching a cubic lattice along two of its orthogonal pairs by two different factors, resulting in a rectangular prism with a r ...
, rhombohedral, and tetragonal. For some of the synthetically produced transuranic elements, available samples have been too small to determine crystal structures.
Occurrence and origin on Earth
Chemical elements may also be categorized by their origin on Earth, with the first 94 considered naturally occurring, while those with atomic numbers beyond 94 have only been produced artificially as the synthetic products of man-made nuclear reactions. Of the 94 naturally occurring elements, 83 are consideredprimordial
Primordial may refer to:
* Primordial era, an era after the Big Bang. See Chronology of the universe
* Primordial sea (a.k.a. primordial ocean, ooze or soup). See Abiogenesis
* Primordial nuclide, nuclides, a few radioactive, that formed before ...
and either stable
A stable is a building in which livestock, especially horses, are kept. It most commonly means a building that is divided into separate stalls for individual animals and livestock. There are many different types of stables in use today; the ...
or weakly radioactive. The remaining 11 naturally occurring elements possess half lives too short for them to have been present at the beginning of the Solar System, and are therefore considered transient elements. Of these 11 transient elements, 5 ( polonium, radon, radium, actinium, and protactinium) are relatively common decay products
In nuclear physics, a decay product (also known as a daughter product, daughter isotope, radio-daughter, or daughter nuclide) is the remaining nuclide left over from radioactive decay. Radioactive decay often proceeds via a sequence of steps (de ...
of thorium and uranium. The remaining 6 transient elements ( technetium, promethium
Promethium is a chemical element with the symbol Pm and atomic number 61. All of its isotopes are radioactive; it is extremely rare, with only about 500–600 grams naturally occurring in Earth's crust at any given time. Promethium is one of onl ...
, astatine, francium
Francium is a chemical element with the symbol Fr and atomic number 87. It is extremely radioactive; its most stable isotope, francium-223 (originally called actinium K after the natural decay chain it appears in), has a half-life of only 22&nb ...
, neptunium, and plutonium) occur only rarely, as products of rare decay modes or nuclear reaction processes involving uranium or other heavy elements.
No radioactive decay has been observed for elements with atomic numbers 1 through 82, except 43 ( technetium) and 61 (promethium
Promethium is a chemical element with the symbol Pm and atomic number 61. All of its isotopes are radioactive; it is extremely rare, with only about 500–600 grams naturally occurring in Earth's crust at any given time. Promethium is one of onl ...
). Observationally stable isotopes of some elements (such as tungsten and lead), however, are predicted to be slightly radioactive with very long half-lives: for example, the half-lives predicted for the observationally stable lead isotopes range from 1035 to 10189 years. Elements with atomic numbers 43, 61, and 83 through 94 are unstable enough that their radioactive decay can readily be detected. Three of these elements, bismuth (element 83), thorium (element 90), and uranium (element 92) have one or more isotopes with half-lives long enough to survive as remnants of the explosive stellar nucleosynthesis
Stellar nucleosynthesis is the creation (nucleosynthesis) of chemical elements by nuclear fusion reactions within stars. Stellar nucleosynthesis has occurred since the original creation of hydrogen, helium and lithium during the Big Bang. As a ...
that produced the heavy elements before the formation of the Solar System. For example, at over 1.9 years, over a billion times longer than the current estimated age of the universe, bismuth-209 has the longest known alpha decay half-life of any naturally occurring element. The very heaviest 24 elements (those beyond plutonium, element 94) undergo radioactive decay with short half-lives and cannot be produced as daughters of longer-lived elements, and thus are not known to occur in nature at all.
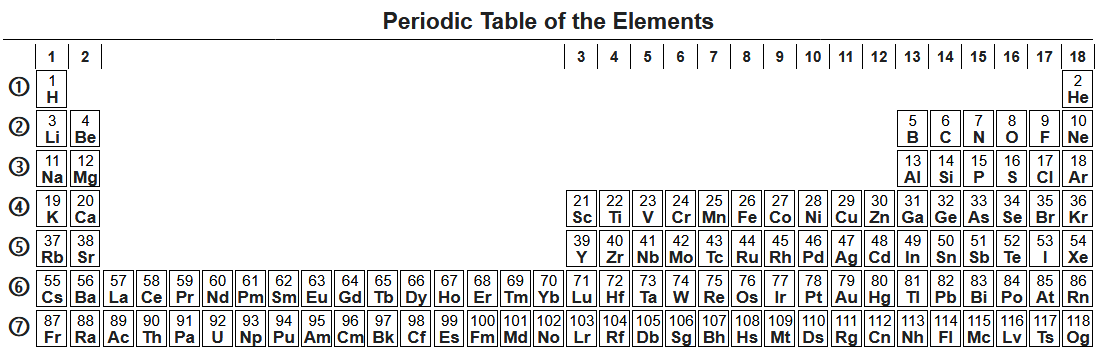
Periodic table
The properties of the chemical elements are often summarized using theperiodic table
The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of ch ...
, which powerfully and elegantly organizes the elements by increasing atomic number into rows ( "periods") in which the columns ( "groups") share recurring ("periodic") physical and chemical properties. The current standard table contains 118 confirmed elements as of 2021.
Although earlier precursors to this presentation exist, its invention is generally credited to the Russian chemist Dmitri Mendeleev
Dmitri Ivanovich Mendeleev (sometimes transliterated as Mendeleyev or Mendeleef) ( ; russian: links=no, Дмитрий Иванович Менделеев, tr. , ; 8 February Old_Style_and_New_Style_dates">O.S._27_January.html" ;"title="O ...
in 1869, who intended the table to illustrate recurring trends in the properties of the elements. The layout of the table has been refined and extended over time as new elements have been discovered and new theoretical models have been developed to explain chemical behavior.
Use of the periodic table is now ubiquitous within the academic discipline of chemistry, providing an extremely useful framework to classify, systematize and compare all the many different forms of chemical behavior. The table has also found wide application in physics, geology, biology, materials science, engineering, agriculture, medicine, nutrition, environmental health, and astronomy. Its principles are especially important in chemical engineering.
Nomenclature and symbols
The various chemical elements are formally identified by their unique atomic numbers, by their accepted names, and by theirsymbols
A symbol is a mark, sign, or word that indicates, signifies, or is understood as representing an idea, object, or relationship. Symbols allow people to go beyond what is known or seen by creating linkages between otherwise very different conc ...
.
Atomic numbers
The known elements have atomic numbers from 1 through 118, conventionally presented asArabic numerals
Arabic numerals are the ten numerical digits: , , , , , , , , and . They are the most commonly used symbols to write Decimal, decimal numbers. They are also used for writing numbers in other systems such as octal, and for writing identifiers ...
. Since the elements can be uniquely sequenced by atomic number, conventionally from lowest to highest (as in a periodic table
The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of ch ...
), sets of elements are sometimes specified by such notation as "through", "beyond", or "from ... through", as in "through iron", "beyond uranium", or "from lanthanum through lutetium". The terms "light" and "heavy" are sometimes also used informally to indicate relative atomic numbers (not densities), as in "lighter than carbon" or "heavier than lead", although technically the weight or mass of atoms of an element (their atomic weights or atomic masses) do not always increase monotonically with their atomic numbers.
Element names
The naming of various substances now known as elements precedes theatomic theory of matter
Atomic theory is the scientific theory that matter is composed of particles called atoms. Atomic theory traces its origins to an ancient philosophical tradition known as atomism. According to this idea, if one were to take a lump of matter an ...
, as names were given locally by various cultures to various minerals, metals, compounds, alloys, mixtures, and other materials, although at the time it was not known which chemicals were elements and which compounds. As they were identified as elements, the existing names for anciently known elements (e.g., gold, mercury, iron) were kept in most countries. National differences emerged over the names of elements either for convenience, linguistic niceties, or nationalism. For a few illustrative examples: German speakers use "Wasserstoff" (water substance) for "hydrogen", "Sauerstoff" (acid substance) for "oxygen" and "Stickstoff" (smothering substance) for "nitrogen", while English and some romance language
The Romance languages, sometimes referred to as Latin languages or Neo-Latin languages, are the various modern languages that evolved from Vulgar Latin. They are the only extant subgroup of the Italic languages in the Indo-European languages, I ...
s use "sodium" for "natrium" and "potassium" for "kalium", and the French, Italians, Greeks, Portuguese and Poles prefer "azote/azot/azoto" (from roots meaning "no life") for "nitrogen".
For purposes of international communication and trade, the official names of the chemical elements both ancient and more recently recognized are decided by the International Union of Pure and Applied Chemistry (IUPAC), which has decided on a sort of international English language, drawing on traditional English names even when an element's chemical symbol is based on a Latin or other traditional word, for example adopting "gold" rather than "aurum" as the name for the 79th element (Au). IUPAC prefers the British spellings " aluminium" and "caesium" over the U.S. spellings "aluminum" and "cesium", and the U.S. "sulfur" over the British "sulphur". However, elements that are practical to sell in bulk in many countries often still have locally used national names, and countries whose national language does not use the Latin alphabet are likely to use the IUPAC element names.
According to IUPAC, chemical elements are not proper nouns in English; consequently, the full name of an element is not routinely capitalized in English, even if derived from a proper noun, as in californium and einsteinium. Isotope names of chemical elements are also uncapitalized if written out, ''e.g.,'' carbon-12
Carbon-12 (12C) is the most abundant of the two stable isotopes of carbon (carbon-13 being the other), amounting to 98.93% of element carbon on Earth; its abundance is due to the triple-alpha process by which it is created in stars. Carbon-12 i ...
or uranium-235. Chemical element ''symbols'' (such as Cf for californium and Es for einsteinium), are always capitalized (see below).
In the second half of the twentieth century, physics laboratories became able to produce nuclei of chemical elements with half-lives too short for an appreciable amount of them to exist at any time. These are also named by IUPAC, which generally adopts the name chosen by the discoverer. This practice can lead to the controversial question of which research group actually discovered an element, a question that delayed the naming of elements with atomic number of 104 and higher for a considerable amount of time. (See element naming controversy).
Precursors of such controversies involved the nationalistic namings of elements in the late 19th century. For example, '' lutetium'' was named in reference to Paris, France. The Germans were reluctant to relinquish naming rights to the French, often calling it ''cassiopeium''. Similarly, the British discoverer of ''niobium
Niobium is a chemical element with chemical symbol Nb (formerly columbium, Cb) and atomic number 41. It is a light grey, crystalline, and ductile transition metal. Pure niobium has a Mohs hardness rating similar to pure titanium, and it has sim ...
'' originally named it ''columbium,'' in reference to the New World. It was used extensively as such by American publications before the international standardization (in 1950).
Chemical symbols
Specific chemical elements
Before chemistry became a science, alchemists had designed arcane symbols for both metals and common compounds. These were however used as abbreviations in diagrams or procedures; there was no concept of atoms combining to form molecules. With his advances in the atomic theory of matter,John Dalton
John Dalton (; 5 or 6 September 1766 – 27 July 1844) was an English chemist, physicist and meteorologist. He is best known for introducing the atomic theory into chemistry, and for his research into colour blindness, which he had. Colour b ...
devised his own simpler symbols, based on circles, to depict molecules.
The current system of chemical notation was invented by Berzelius. In this typographical system, chemical symbols are not mere abbreviations—though each consists of letters of the Latin alphabet. They are intended as universal symbols for people of all languages and alphabets.
The first of these symbols were intended to be fully universal. Since Latin was the common language of science at that time, they were abbreviations based on the Latin names of metals. Cu comes from cuprum, Fe comes from ferrum, Ag from argentum. The symbols were not followed by a period (full stop) as with abbreviations. Later chemical elements were also assigned unique chemical symbols, based on the name of the element, but not necessarily in English. For example, sodium has the chemical symbol 'Na' after the Latin ''natrium''. The same applies to "Fe" (ferrum) for iron, "Hg" (hydrargyrum) for mercury
Mercury commonly refers to:
* Mercury (planet), the nearest planet to the Sun
* Mercury (element), a metallic chemical element with the symbol Hg
* Mercury (mythology), a Roman god
Mercury or The Mercury may also refer to:
Companies
* Merc ...
, "Sn" (stannum) for tin, "Au" (aurum) for gold, "Ag" (argentum) for silver, "Pb" (plumbum) for lead, "Cu" (cuprum) for copper, and "Sb" (stibium) for antimony. "W" (wolfram) for tungsten ultimately derives from German, "K" (kalium) for potassium ultimately from Arabic.
Chemical symbols are understood internationally when element names might require translation. There have sometimes been differences in the past. For example, Germans in the past have used "J" (for the alternate name Jod) for iodine, but now use "I" and "Iod".
The first letter of a chemical symbol is always capitalized, as in the preceding examples, and the subsequent letters, if any, are always lower case (small letters). Thus, the symbols for californium and einsteinium are Cf and Es.
General chemical symbols
There are also symbols in chemical equations for groups of chemical elements, for example in comparative formulas. These are often a single capital letter, and the letters are reserved and not used for names of specific elements. For example, an "X" indicates a variable group (usually ahalogen
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this group is ...
) in a class of compounds, while "R" is a radical
Radical may refer to:
Politics and ideology Politics
*Radical politics, the political intent of fundamental societal change
*Radicalism (historical), the Radical Movement that began in late 18th century Britain and spread to continental Europe and ...
, meaning a compound structure such as a hydrocarbon chain. The letter "Q" is reserved for "heat" in a chemical reaction. "Y" is also often used as a general chemical symbol, although it is also the symbol of yttrium. "Z" is also frequently used as a general variable group. "E" is used in organic chemistry to denote an electron-withdrawing group
In chemistry, an electron-withdrawing group (EWG) is a substituent that has some of the following kinetic and thermodynamic implications:
*with regards to electron transfer, electron-withdrawing groups enhance the oxidizing power tendency of the ...
or an electrophile; similarly "Nu" denotes a nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
. "L" is used to represent a general ligand in inorganic and organometallic chemistry
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and so ...
. "M" is also often used in place of a general metal.
At least two additional, two-letter generic chemical symbols are also in informal usage, "Ln" for any lanthanide
The lanthanide () or lanthanoid () series of chemical elements comprises the 15 metallic chemical elements with atomic numbers 57–71, from lanthanum through lutetium. These elements, along with the chemically similar elements scandium and yttr ...
element and "An" for any actinide element. "Rg" was formerly used for any rare gas element, but the group of rare gases has now been renamed noble gases and the symbol "Rg" has now been assigned to the element roentgenium.
Isotope symbols
Isotopes are distinguished by the atomic mass number (total protons and neutrons) for a particular isotope of an element, with this number combined with the pertinent element's symbol. IUPAC prefers that isotope symbols be written in superscript notation when practical, for example 12C and 235U. However, other notations, such as carbon-12 and uranium-235, or C-12 and U-235, are also used. As a special case, the three naturally occurring isotopes of the element hydrogen are often specified as H for 1H ( protium), D for 2H ( deuterium), and T for 3H ( tritium). This convention is easier to use in chemical equations, replacing the need to write out the mass number for each atom. For example, the formula for heavy water may be written D2O instead of 2H2O.Origin of the elements
 Only about 4% of the total mass of the universe is made of atoms or ions, and thus represented by chemical elements. This fraction is about 15% of the total matter, with the remainder of the matter (85%) being dark matter. The nature of dark matter is unknown, but it is not composed of atoms of chemical elements because it contains no protons, neutrons, or electrons. (The remaining non-matter part of the mass of the universe is composed of the even less well understood dark energy).
The 94 naturally occurring chemical elements were produced by at least four classes of astrophysical process. Most of the hydrogen, helium and a very small quantity of lithium were produced in the first few minutes of the
Only about 4% of the total mass of the universe is made of atoms or ions, and thus represented by chemical elements. This fraction is about 15% of the total matter, with the remainder of the matter (85%) being dark matter. The nature of dark matter is unknown, but it is not composed of atoms of chemical elements because it contains no protons, neutrons, or electrons. (The remaining non-matter part of the mass of the universe is composed of the even less well understood dark energy).
The 94 naturally occurring chemical elements were produced by at least four classes of astrophysical process. Most of the hydrogen, helium and a very small quantity of lithium were produced in the first few minutes of the Big Bang
The Big Bang event is a physical theory that describes how the universe expanded from an initial state of high density and temperature. Various cosmological models of the Big Bang explain the evolution of the observable universe from the ...
. This Big Bang nucleosynthesis happened only once; the other processes are ongoing. Nuclear fusion inside stars produces elements through stellar nucleosynthesis
Stellar nucleosynthesis is the creation (nucleosynthesis) of chemical elements by nuclear fusion reactions within stars. Stellar nucleosynthesis has occurred since the original creation of hydrogen, helium and lithium during the Big Bang. As a ...
, including all elements from carbon to iron in atomic number. Elements higher in atomic number than iron, including heavy elements like uranium and plutonium, are produced by various forms of explosive nucleosynthesis in supernova
A supernova is a powerful and luminous explosion of a star. It has the plural form supernovae or supernovas, and is abbreviated SN or SNe. This transient astronomical event occurs during the last evolutionary stages of a massive star or when ...
e and neutron star mergers. The light elements lithium, beryllium and boron
Boron is a chemical element with the symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the ''boron group'' it has th ...
are produced mostly through cosmic ray spallation (fragmentation induced by cosmic rays) of carbon, nitrogen, and oxygen.
During the early phases of the Big Bang, nucleosynthesis of hydrogen nuclei resulted in the production of hydrogen-1 ( protium, 1H) and helium-4 (4He), as well as a smaller amount of deuterium (2H) and very minuscule amounts (on the order of 10−10) of lithium and beryllium. Even smaller amounts of boron may have been produced in the Big Bang, since it has been observed in some very old stars, while carbon has not. No elements heavier than boron were produced in the Big Bang. As a result, the primordial abundance of atoms (or ions) consisted of roughly 75% 1H, 25% 4He, and 0.01% deuterium, with only tiny traces of lithium, beryllium, and perhaps boron. Subsequent enrichment of galactic halos occurred due to stellar nucleosynthesis and supernova nucleosynthesis
Supernova nucleosynthesis is the nucleosynthesis of chemical elements in supernova explosions.
In sufficiently massive stars, the nucleosynthesis by fusion of lighter elements into heavier ones occurs during sequential hydrostatic burning processe ...
. However, the element abundance in intergalactic space can still closely resemble primordial conditions, unless it has been enriched by some means.
nuclear transmutation
Nuclear transmutation is the conversion of one chemical element or an isotope into another chemical element. Nuclear transmutation occurs in any process where the number of protons or neutrons in the nucleus of an atom is changed.
A transmutatio ...
processes. These include some produced by cosmic rays or other nuclear reactions (see cosmogenic and nucleogenic nuclides), and others produced as decay product
In nuclear physics, a decay product (also known as a daughter product, daughter isotope, radio-daughter, or daughter nuclide) is the remaining nuclide left over from radioactive decay. Radioactive decay often proceeds via a sequence of steps ( ...
s of long-lived primordial nuclide
In geochemistry, geophysics and nuclear physics, primordial nuclides, also known as primordial isotopes, are nuclides found on Earth that have existed in their current form since before Earth was formed. Primordial nuclides were present in the ...
s. For example, trace (but detectable) amounts of carbon-14
Carbon-14, C-14, or radiocarbon, is a radioactive isotope of carbon with an atomic nucleus containing 6 protons and 8 neutrons. Its presence in organic materials is the basis of the radiocarbon dating method pioneered by Willard Libby and coll ...
(14C) are continually produced in the atmosphere by cosmic rays impacting nitrogen atoms, and argon-40 (40Ar) is continually produced by the decay of primordially occurring but unstable potassium-40 (40K). Also, three primordially occurring but radioactive actinides, thorium, uranium, and plutonium, decay through a series of recurrently produced but unstable radioactive elements such as radium and radon, which are transiently present in any sample of these metals or their ores or compounds. Three other radioactive elements, technetium, promethium
Promethium is a chemical element with the symbol Pm and atomic number 61. All of its isotopes are radioactive; it is extremely rare, with only about 500–600 grams naturally occurring in Earth's crust at any given time. Promethium is one of onl ...
, and neptunium, occur only incidentally in natural materials, produced as individual atoms by nuclear fission
Nuclear fission is a reaction in which the nucleus of an atom splits into two or more smaller nuclei. The fission process often produces gamma photons, and releases a very large amount of energy even by the energetic standards of radio ...
of the nuclei of various heavy elements or in other rare nuclear processes.
In addition to the 94 naturally occurring elements, several artificial element
A synthetic element is one of 24 known chemical elements that do not occur naturally on Earth: they have been created by human manipulation of fundamental particles in a nuclear reactor, a particle accelerator, or the explosion of an atomic bomb; ...
s have been produced by human nuclear physics technology. , these experiments have produced all elements up to atomic number 118.
Abundance
The following graph (note log scale) shows the abundance of elements in our Solar System. The table shows the twelve most common elements in our galaxy (estimated spectroscopically), as measured in parts per million, by mass. Nearby galaxies that have evolved along similar lines have a corresponding enrichment of elements heavier than hydrogen and helium. The more distant galaxies are being viewed as they appeared in the past, so their abundances of elements appear closer to the primordial mixture. As physical laws and processes appear common throughout thevisible universe
The observable universe is a ball-shaped region of the universe comprising all matter that can be observed from Earth or its space-based telescopes and exploratory probes at the present time, because the electromagnetic radiation from these obje ...
, however, scientist expect that these galaxies evolved elements in similar abundance.
The abundance of elements in the Solar System is in keeping with their origin from nucleosynthesis in the Big Bang
The Big Bang event is a physical theory that describes how the universe expanded from an initial state of high density and temperature. Various cosmological models of the Big Bang explain the evolution of the observable universe from the ...
and a number of progenitor supernova stars. Very abundant hydrogen and helium are products of the Big Bang, but the next three elements are rare since they had little time to form in the Big Bang and are not made in stars (they are, however, produced in small quantities by the breakup of heavier elements in interstellar dust, as a result of impact by cosmic rays). Beginning with carbon, elements are produced in stars by buildup from alpha particles (helium nuclei), resulting in an alternatingly larger abundance of elements with even atomic numbers (these are also more stable). In general, such elements up to iron are made in large stars in the process of becoming supernova
A supernova is a powerful and luminous explosion of a star. It has the plural form supernovae or supernovas, and is abbreviated SN or SNe. This transient astronomical event occurs during the last evolutionary stages of a massive star or when ...
s. Iron-56 is particularly common, since it is the most stable element that can easily be made from alpha particles (being a product of decay of radioactive nickel-56, ultimately made from 14 helium nuclei). Elements heavier than iron are made in energy-absorbing processes in large stars, and their abundance in the universe (and on Earth) generally decreases with their atomic number.
The abundance of the chemical elements on Earth varies from air to crust to ocean, and in various types of life. The abundance of elements in Earth's crust differs from that in the Solar System (as seen in the Sun and heavy planets like Jupiter) mainly in selective loss of the very lightest elements (hydrogen and helium) and also volatile neon, carbon (as hydrocarbons), nitrogen and sulfur, as a result of solar heating in the early formation of the solar system. Oxygen, the most abundant Earth element by mass, is retained on Earth by combination with silicon. Aluminum at 8% by mass is more common in the Earth's crust than in the universe and solar system, but the composition of the far more bulky mantle, which has magnesium and iron in place of aluminum (which occurs there only at 2% of mass) more closely mirrors the elemental composition of the solar system, save for the noted loss of volatile elements to space, and loss of iron which has migrated to the Earth's core.
The composition of the human body, by contrast, more closely follows the composition of seawater—save that the human body has additional stores of carbon and nitrogen necessary to form the proteins and nucleic acid
Nucleic acids are biopolymers, macromolecules, essential to all known forms of life. They are composed of nucleotides, which are the monomers made of three components: a 5-carbon sugar, a phosphate group and a nitrogenous base. The two main cl ...
s, together with phosphorus in the nucleic acids and energy transfer molecule adenosine triphosphate (ATP) that occurs in the cells of all living organisms. Certain kinds of organisms require particular additional elements, for example the magnesium in chlorophyll
Chlorophyll (also chlorophyl) is any of several related green pigments found in cyanobacteria and in the chloroplasts of algae and plants. Its name is derived from the Greek words , ("pale green") and , ("leaf"). Chlorophyll allow plants to a ...
in green plants, the calcium in mollusc shells, or the iron in the hemoglobin in vertebrate animals' red blood cells.

History
Evolving definitions
The concept of an "element" as an undivisible substance has developed through three major historical phases: Classical definitions (such as those of the ancient Greeks), chemical definitions, and atomic definitions.Classical definitions
Ancient philosophy posited a set ofclassical element
Classical elements typically refer to earth, water, air, fire, and (later) aether which were proposed to explain the nature and complexity of all matter in terms of simpler substances. Ancient cultures in Greece, Tibet, and India had simil ...
s to explain observed patterns in nature. These ''elements'' originally referred to '' earth'', '' water'', '' air'' and '' fire'' rather than the chemical elements of modern science.
The term 'elements' (''stoicheia'') was first used by the Greek philosopher Plato in about 360 BCE in his dialogue Timaeus Timaeus (or Timaios) is a Greek name. It may refer to:
* ''Timaeus'' (dialogue), a Socratic dialogue by Plato
*Timaeus of Locri, 5th-century BC Pythagorean philosopher, appearing in Plato's dialogue
*Timaeus (historian) (c. 345 BC-c. 250 BC), Greek ...
, which includes a discussion of the composition of inorganic and organic bodies and is a speculative treatise on chemistry. Plato believed the elements introduced a century earlier by Empedocles were composed of small polyhedral forms: tetrahedron (fire), octahedron (air), icosahedron
In geometry, an icosahedron ( or ) is a polyhedron with 20 faces. The name comes and . The plural can be either "icosahedra" () or "icosahedrons".
There are infinitely many non- similar shapes of icosahedra, some of them being more symmetrica ...
(water), and cube
In geometry, a cube is a three-dimensional solid object bounded by six square faces, facets or sides, with three meeting at each vertex. Viewed from a corner it is a hexagon and its net is usually depicted as a cross.
The cube is the only r ...
(earth).
Aristotle, c. 350 BCE, also used the term ''stoicheia'' and added a fifth element called aether Aether, æther or ether may refer to:
Metaphysics and mythology
* Aether (classical element), the material supposed to fill the region of the universe above the terrestrial sphere
* Aether (mythology), the personification of the "upper sky", sp ...
, which formed the heavens. Aristotle defined an element as:
Chemical definitions
In 1661, Robert Boyle proposed his theory of corpuscularism which favoured the analysis of matter as constituted by irreducible units of matter (atoms) and, choosing to side with neither Aristotle's view of the four elements norParacelsus
Paracelsus (; ; 1493 – 24 September 1541), born Theophrastus von Hohenheim (full name Philippus Aureolus Theophrastus Bombastus von Hohenheim), was a Swiss physician, alchemist, lay theologian, and philosopher of the German Renaissance.
He w ...
' view of three fundamental elements, left open the question of the number of elements. The first modern list of chemical elements was given in Antoine Lavoisier's 1789 '' Elements of Chemistry'', which contained thirty-three elements, including light and caloric
Caloric is a brand of kitchen appliances, which dates back to 1903.
History
Caloric Corporation began as the Klein Stove Company in Philadelphia in 1890. The Caloric brand was introduced in 1903. It was reorganized in 1946 as the Caloric Stove C ...
. By 1818, Jöns Jakob Berzelius had determined atomic weights for forty-five of the forty-nine then-accepted elements. Dmitri Mendeleev
Dmitri Ivanovich Mendeleev (sometimes transliterated as Mendeleyev or Mendeleef) ( ; russian: links=no, Дмитрий Иванович Менделеев, tr. , ; 8 February Old_Style_and_New_Style_dates">O.S._27_January.html" ;"title="O ...
had sixty-six elements in his periodic table
The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of ch ...
of 1869.
 From Boyle until the early 20th century, an element was defined as a pure substance that could not be decomposed into any simpler substance. Put another way, a chemical element cannot be transformed into other chemical elements by chemical processes. Elements during this time were generally distinguished by their atomic weights, a property measurable with fair accuracy by available analytical techniques.
From Boyle until the early 20th century, an element was defined as a pure substance that could not be decomposed into any simpler substance. Put another way, a chemical element cannot be transformed into other chemical elements by chemical processes. Elements during this time were generally distinguished by their atomic weights, a property measurable with fair accuracy by available analytical techniques.
Atomic definitions
The 1913 discovery by English physicist Henry Moseley that the nuclear charge is the physical basis for an atom's atomic number, further refined when the nature of protons andneutrons
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons behave ...
became appreciated, eventually led to the current definition of an element based on atomic number (number of protons per atomic nucleus). The use of atomic numbers, rather than atomic weights, to distinguish elements has greater predictive value (since these numbers are integers), and also resolves some ambiguities in the chemistry-based view due to varying properties of isotopes and allotropes within the same element. Currently, IUPAC defines an element to exist if it has isotopes with a lifetime longer than the 10−14 seconds it takes the nucleus to form an electronic cloud.
By 1914, seventy-two elements were known, all naturally occurring. The remaining naturally occurring elements were discovered or isolated in subsequent decades, and various additional elements have also been produced synthetically, with much of that work pioneered by Glenn T. Seaborg. In 1955, element 101 was discovered and named mendelevium in honor of D.I. Mendeleev, the first to arrange the elements in a periodic manner.
Discovery and recognition of various elements
Ten materials familiar to various prehistoric cultures are now known to be chemical elements: Carbon, copper, gold, iron, lead,mercury
Mercury commonly refers to:
* Mercury (planet), the nearest planet to the Sun
* Mercury (element), a metallic chemical element with the symbol Hg
* Mercury (mythology), a Roman god
Mercury or The Mercury may also refer to:
Companies
* Merc ...
, silver, sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula ...
, tin, and zinc. Three additional materials now accepted as elements, arsenic, antimony, and bismuth, were recognized as distinct substances prior to 1500 AD. Phosphorus, cobalt, and platinum were isolated before 1750.
Most of the remaining naturally occurring chemical elements were identified and characterized by 1900, including:
* Such now-familiar industrial materials as aluminium, silicon, nickel, chromium
Chromium is a chemical element with the symbol Cr and atomic number 24. It is the first element in group 6. It is a steely-grey, lustrous, hard, and brittle transition metal.
Chromium metal is valued for its high corrosion resistance and hardne ...
, magnesium, and tungsten
* Reactive metals such as lithium, sodium, potassium, and calcium
* The halogen
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this group is ...
s fluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reacti ...
, chlorine, bromine, and iodine
Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , and boils to a vi ...
* Gases such as hydrogen, oxygen, nitrogen, helium, argon, and neon
Neon is a chemical element with the symbol Ne and atomic number 10. It is a noble gas. Neon is a colorless, odorless, inert monatomic gas under standard conditions, with about two-thirds the density of air. It was discovered (along with krypton ...
* Most of the rare-earth elements, including cerium, lanthanum, gadolinium, and neodymium.
* The more common radioactive elements, including uranium, thorium, radium, and radon
Elements isolated or produced since 1900 include:
* The three remaining undiscovered regularly occurring stable natural elements: hafnium, lutetium, and rhenium
* Plutonium, which was first produced synthetically in 1940 by Glenn T. Seaborg, but is now also known from a few long-persisting natural occurrences
* The three incidentally occurring natural elements ( neptunium, promethium
Promethium is a chemical element with the symbol Pm and atomic number 61. All of its isotopes are radioactive; it is extremely rare, with only about 500–600 grams naturally occurring in Earth's crust at any given time. Promethium is one of onl ...
, and technetium), which were all first produced synthetically but later discovered in trace amounts in certain geological samples
* Four scarce decay products of uranium or thorium ( astatine, francium
Francium is a chemical element with the symbol Fr and atomic number 87. It is extremely radioactive; its most stable isotope, francium-223 (originally called actinium K after the natural decay chain it appears in), has a half-life of only 22&nb ...
, actinium, and protactinium), and
* Various synthetic transuranic elements, beginning with americium
Americium is a synthetic radioactive chemical element with the symbol Am and atomic number 95. It is a transuranic member of the actinide series, in the periodic table located under the lanthanide element europium, and thus by analogy was na ...
and curium
Curium is a transuranic, radioactive chemical element with the symbol Cm and atomic number 96. This actinide element was named after eminent scientists Marie and Pierre Curie, both known for their research on radioactivity. Curium was first inte ...
Recently discovered elements
The first transuranium element (element with atomic number greater than 92) discovered was neptunium in 1940. Since 1999 claims for the discovery of new elements have been considered by the IUPAC/IUPAP Joint Working Party. As of January 2016, all 118 elements have been confirmed as discovered by IUPAC. The discovery of element 112 was acknowledged in 2009, and the name ''copernicium'' and the atomic symbol ''Cn'' were suggested for it. The name and symbol were officially endorsed by IUPAC on 19 February 2010. The heaviest element that is believed to have been synthesized to date is element 118, oganesson, on 9 October 2006, by the Flerov Laboratory of Nuclear Reactions in Dubna, Russia. Tennessine, element 117 was the latest element claimed to be discovered, in 2009. On 28 November 2016, scientists at the IUPAC officially recognized the names for four of the newest chemical elements, with atomic numbers 113, 115, 117, and 118.List of the 118 known chemical elements
The following sortable table shows the 118 known chemical elements. * Atomic number, Element, and Symbol all serve independently as unique identifiers. * Element names are those accepted by IUPAC. * Block indicates the periodic table block for each element: red = s-block, yellow = p-block, blue = d-block, green = f-block. * Group and period refer to an element's position in theperiodic table
The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of ch ...
. Group numbers here show the currently accepted numbering; for older numberings, see Group (periodic table).
See also
* Biological roles of the elements * Chemical database * Discovery of the chemical elements *Element collecting
Element collecting is the hobby of collecting the chemical elements. Many element collectors simply enjoy finding peculiar uses of chemical elements. Others enjoy studying the properties of the elements, possibly engaging in amateur chemistry, a ...
* Fictional element
This list contains fictional chemical elements, materials, isotopes or subatomic particles that either a) play a major role in a notable work of fiction, b) are common to several unrelated works, or c) are discussed in detail by independent sou ...
* Goldschmidt classification
* Island of stability
* List of nuclides
* List of the elements' densities
* Mineral (nutrient)
* Periodic Systems of Small Molecules
* Prices of chemical elements
This is a list of prices of chemical elements. Listed here are mainly average market prices for bulk trade of commodities. Data on elements' abundance in Earth's crust is added for comparison.
As of 2020, the most expensive non- synthetic element ...
* Systematic element name
* Table of nuclides
* Timeline of chemical element discoveries
* Roles of chemical elements
References
Further reading
* * * * * * * *: XML on-line corrected version: created by M. Nic, J. Jirat, B. Kosata; updates compiled by A. JenkinsExternal links
Videos for each element
by the University of Nottingham
"Chemical Elements"
''In Our Time'', BBC Radio 4 discussion with Paul Strathern, Mary Archer and John Murrell (25 May 2000) {{Authority control Chemistry