Unbibium on:
[Wikipedia]
[Google]
[Amazon]
Unbibium, also known as element 122 or eka-thorium, is a hypothetical
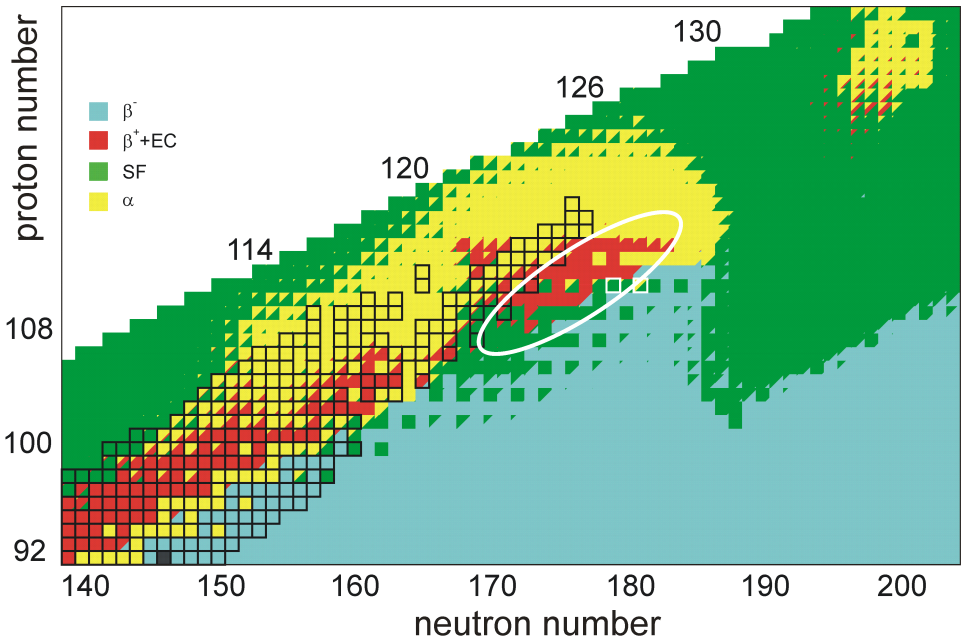 Every element from
Every element from
 The stability of nuclei decreases greatly with the increase in atomic number after
The stability of nuclei decreases greatly with the increase in atomic number after  A quantum tunneling model predicts the alpha-decay half-lives of unbibium isotopes 284–322Ubb to be on the order of microseconds or less for all isotopes lighter than 315Ubb, highlighting a significant challenge in experimental observation of this element. This is consistent with many predictions, though the exact location of the 1 microsecond border varies by model. Additionally, spontaneous fission is expected to become a major decay mode in this region, with half-lives on the order of femtoseconds predicted for some even–even isotopes due to minimal hindrance resulting from nucleon pairing and loss of stabilizing effects farther away from magic numbers. A 2016 calculation on the half-lives and probable decay chains of isotopes 280–339Ubb yields corroborating results: 280–297Ubb will be proton unbound and possibly decay by
A quantum tunneling model predicts the alpha-decay half-lives of unbibium isotopes 284–322Ubb to be on the order of microseconds or less for all isotopes lighter than 315Ubb, highlighting a significant challenge in experimental observation of this element. This is consistent with many predictions, though the exact location of the 1 microsecond border varies by model. Additionally, spontaneous fission is expected to become a major decay mode in this region, with half-lives on the order of femtoseconds predicted for some even–even isotopes due to minimal hindrance resulting from nucleon pairing and loss of stabilizing effects farther away from magic numbers. A 2016 calculation on the half-lives and probable decay chains of isotopes 280–339Ubb yields corroborating results: 280–297Ubb will be proton unbound and possibly decay by
Chemistry-Blog: Independent analysis of Marinov's 122 claimChart of the Nuclides 2014
{{Extended periodic table (by Fricke, 32 columns, compact)
chemical element
A chemical element is a chemical substance whose atoms all have the same number of protons. The number of protons is called the atomic number of that element. For example, oxygen has an atomic number of 8: each oxygen atom has 8 protons in its ...
; it has placeholder symbol Ubb and atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of its atomic nucleus. For ordinary nuclei composed of protons and neutrons, this is equal to the proton number (''n''p) or the number of pro ...
122. ''Unbibium'' and ''Ubb'' are the temporary systematic IUPAC name and symbol respectively, which are used until the element is discovered, confirmed, and a permanent name is decided upon. In the periodic table
The periodic table, also known as the periodic table of the elements, is an ordered arrangement of the chemical elements into rows (" periods") and columns (" groups"). It is an icon of chemistry and is widely used in physics and other s ...
of the elements, it is expected to follow unbiunium
Unbiunium, also known as eka-actinium or element 121, is a hypothetical chemical element; it has symbol Ubu and atomic number 121. ''Unbiunium'' and ''Ubu'' are the temporary systematic IUPAC name and symbol respectively, which are used until th ...
as the second element of the superactinides
An extended periodic table theorizes about chemical elements beyond those currently known and proven. The element with the highest atomic number known is oganesson (''Z'' = 118), which completes the seventh period (row) in the perio ...
and the fourth element of the 8th period
Period may refer to:
Common uses
* Period (punctuation)
* Era, a length or span of time
*Menstruation, commonly referred to as a "period"
Arts, entertainment, and media
* Period (music), a concept in musical composition
* Periodic sentence (o ...
. Similarly to unbiunium, it is expected to fall within the range of the island of stability
In nuclear physics, the island of stability is a predicted set of isotopes of superheavy elements that may have considerably longer half-lives than known isotopes of these elements. It is predicted to appear as an "island" in the chart of nuclid ...
, potentially conferring additional stability on some isotopes, especially 306Ubb which is expected to have a magic number of neutrons (184).
Despite several attempts, unbibium has not yet been synthesized, nor have any naturally occurring isotopes been found to exist. There are currently no plans to attempt to synthesize unbibium. In 2008, it was claimed to have been discovered in natural thorium samples, but that claim has now been dismissed by recent repetitions of the experiment using more accurate techniques.
Chemically, unbibium is expected to show some resemblance to cerium
Cerium is a chemical element; it has Chemical symbol, symbol Ce and atomic number 58. It is a hardness, soft, ductile, and silvery-white metal that tarnishes when exposed to air. Cerium is the second element in the lanthanide series, and while it ...
and thorium
Thorium is a chemical element; it has symbol Th and atomic number 90. Thorium is a weakly radioactive light silver metal which tarnishes olive grey when it is exposed to air, forming thorium dioxide; it is moderately soft, malleable, and ha ...
. However, relativistic effects may cause some of its properties to differ; for example, it is expected to have a ground state electron configuration of [ Og] 7d1 8s2 8p1 or g8s2 8p2, despite its predicted position in the g-block superactinide series.
Introduction
History
Synthesis attempts
Fusion-evaporation
Two attempts were made to synthesize unbibium in the 1970s, both propelled by early predictions on the island of stability at ''N'' = 184 and ''Z'' > 120, and in particular whether superheavy elements could potentially be naturally occurring. The first attempts to synthesize unbibium were performed in 1972 by Flerov et al. at theJoint Institute for Nuclear Research
The Joint Institute for Nuclear Research (JINR, ), in Dubna, Moscow Oblast (110 km north of Moscow), Russia, is an international research center for nuclear sciences, with 5,500 staff members including 1,200 researchers holding over 1,000 ...
(JINR), using the heavy-ion induced hot fusion reactions:
: + → * → no atoms
Another unsuccessful attempt to synthesize unbibium was carried out in 1978 at the GSI Helmholtz Center, where a natural erbium
Erbium is a chemical element; it has Symbol (chemistry), symbol Er and atomic number 68. A silvery-white solid metal when artificially isolated, natural erbium is always found in chemical combination with other elements. It is a lanthanide, a rare- ...
target was bombarded with xenon-136
Naturally occurring xenon (54Xe) consists of seven stable isotopes and two very long-lived isotopes. Double electron capture has been observed in 124Xe (half-life ) and double beta decay in 136Xe (half-life ), which are among the longest measured ...
ions:
: + → * → no atoms
No atoms were detected and a yield limit of 5 nb (5,000 pb) was measured. Current results (see flerovium
Flerovium is a synthetic chemical element; it has symbol Fl and atomic number 114. It is an extremely radioactive, superheavy element, named after the Flerov Laboratory of Nuclear Reactions of the Joint Institute for Nuclear Research in Du ...
) have shown that the sensitivity of these experiments were too low by at least 3 orders of magnitude. In particular, the reaction between 170Er and 136Xe was expected to yield alpha emitters with half-lives of microseconds that would decay down to isotopes of flerovium
Flerovium is a synthetic chemical element; it has symbol Fl and atomic number 114. It is an extremely radioactive, superheavy element, named after the Flerov Laboratory of Nuclear Reactions of the Joint Institute for Nuclear Research in Du ...
with half-lives perhaps increasing up to several hours, as flerovium is predicted to lie near the center of the island of stability. After twelve hours of irradiation, nothing was found in this reaction. Following a similar unsuccessful attempt to synthesize unbiunium
Unbiunium, also known as eka-actinium or element 121, is a hypothetical chemical element; it has symbol Ubu and atomic number 121. ''Unbiunium'' and ''Ubu'' are the temporary systematic IUPAC name and symbol respectively, which are used until th ...
from 238U and 65Cu, it was concluded that half-lives of superheavy nuclei must be less than one microsecond or the cross sections are very small. More recent research into synthesis of superheavy elements suggests that both conclusions are true.
In 2000, the Gesellschaft für Schwerionenforschung
The GSI Helmholtz Centre for Heavy Ion Research () is a federally and state co-funded heavy ion () research center in Darmstadt, Germany. It was founded in 1969 as the Society for Heavy Ion Research (), abbreviated GSI, to conduct research on ...
(GSI) Helmholtz Center for Heavy Ion Research performed a very similar experiment with much higher sensitivity:
: + → * → no atoms
These results indicate that the synthesis of such heavier elements remains a significant challenge and further improvements of beam intensity and experimental efficiency is required. The sensitivity should be increased to 1 fb in the future for more quality results.
Compound nucleus fission
Several experiments studying the fission characteristics of various superheavy compound nuclei such as 306Ubb were performed between 2000 and 2004 at the Flerov Laboratory of Nuclear Reactions. Two nuclear reactions were used, namely 248Cm + 58Fe and 242Pu + 64Ni. The results reveal how superheavy nuclei fission predominantly by expellingclosed shell
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon ato ...
nuclei such as 132Sn (''Z'' = 50, ''N'' = 82). It was also found that the yield for the fusion-fission pathway was similar between 48Ca and 58Fe projectiles, suggesting a possible future use of 58Fe projectiles in superheavy element formation.
Claimed discovery as a naturally occurring element
In 2008, a group led by Israeli physicist Amnon Marinov at theHebrew University of Jerusalem
The Hebrew University of Jerusalem (HUJI; ) is an Israeli public university, public research university based in Jerusalem. Co-founded by Albert Einstein and Chaim Weizmann in July 1918, the public university officially opened on 1 April 1925. ...
claimed to have found single atoms of unbibium-292 in naturally occurring thorium
Thorium is a chemical element; it has symbol Th and atomic number 90. Thorium is a weakly radioactive light silver metal which tarnishes olive grey when it is exposed to air, forming thorium dioxide; it is moderately soft, malleable, and ha ...
deposits at an abundance of between 10−11 and 10−12 relative to thorium. This was the first time in 69 years that a new element had been claimed to be discovered in nature, after Marguerite Perey
Marguerite Catherine Perey (19 October 1909 – 13 May 1975) was a French physicist and a student of Marie Curie. In 1939, Perey discovered the element francium by purifying samples of lanthanum that contained actinium. In 1962, she was the fi ...
's 1939 discovery of francium
Francium is a chemical element; it has symbol Fr and atomic number 87. It is extremely radioactive; its most stable isotope, francium-223 (originally called '' actinium K'' after the natural decay chain in which it appears), has a half-l ...
. The claim of Marinov ''et al.'' was criticized by the scientific community, and Marinov says he has submitted the article to the journals ''Nature
Nature is an inherent character or constitution, particularly of the Ecosphere (planetary), ecosphere or the universe as a whole. In this general sense nature refers to the Scientific law, laws, elements and phenomenon, phenomena of the physic ...
'' and ''Nature Physics
''Nature Physics'' is a monthly peer-reviewed scientific journal published by Nature Portfolio. It was first published in October 2005 (volume 1, issue 1). The chief editor is David Abergel.
Scope
''Nature Physics'' publishes both pure and appli ...
'' but both turned it down without sending it for peer review. The unbibium-292 atoms were claimed to be superdeformed or hyperdeformed isomers
In chemistry, isomers are molecules or polyatomic ions with identical molecular formula – that is, the same number of atoms of each element – but distinct arrangements of atoms in space. ''Isomerism'' refers to the existence or possibili ...
, with a half-life of at least 100 million years.
A criticism of the technique, previously used in purportedly identifying lighter thorium
Thorium is a chemical element; it has symbol Th and atomic number 90. Thorium is a weakly radioactive light silver metal which tarnishes olive grey when it is exposed to air, forming thorium dioxide; it is moderately soft, malleable, and ha ...
isotopes by mass spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a ''mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is used ...
, was published in ''Physical Review C
Physical may refer to:
*Physical examination
In a physical examination, medical examination, clinical examination, or medical checkup, a medical practitioner examines a patient for any possible medical signs or symptoms of a Disease, medical co ...
'' in 2008. A rebuttal by the Marinov group was published in ''Physical Review C'' after the published comment.
A repeat of the thorium experiment using the superior method of accelerator mass spectrometry
Accelerator mass spectrometry (AMS) is a form of mass spectrometry that accelerates ions to extraordinarily high kinetic energies before mass analysis. The special strength of AMS among the different methods of mass spectrometry is its ability t ...
(AMS) failed to confirm the results, despite a 100-fold better sensitivity. This result throws considerable doubt on the results of the Marinov collaboration with regards to their claims of long-lived isotopes of thorium
Thorium is a chemical element; it has symbol Th and atomic number 90. Thorium is a weakly radioactive light silver metal which tarnishes olive grey when it is exposed to air, forming thorium dioxide; it is moderately soft, malleable, and ha ...
, roentgenium
Roentgenium () is a synthetic chemical element; it has symbol Rg and atomic number 111. It is extremely radioactive and can only be created in a laboratory. The most stable known isotope, roentgenium-282, has a half-life of 130 seconds, althoug ...
, and unbibium. Current understanding of superheavy elements indicates that it is very unlikely for any traces of unbibium to persist in natural thorium samples.
Naming
Using Mendeleev's nomenclature for unnamed and undiscovered elements, unbibium should instead be known as ''eka-thorium
Thorium is a chemical element; it has symbol Th and atomic number 90. Thorium is a weakly radioactive light silver metal which tarnishes olive grey when it is exposed to air, forming thorium dioxide; it is moderately soft, malleable, and ha ...
''. After the recommendations of the IUPAC in 1979, the element has since been largely referred to as ''unbibium'' with the atomic symbol of (''Ubb''), as its temporary name until the element is officially discovered and synthesized, and a permanent name is decided on. Scientists largely ignore this naming convention, and instead simply refer to unbibium as "element 122" with the symbol of (''122''), or sometimes even ''E122'' or ''122''.
Prospects for future synthesis
 Every element from
Every element from mendelevium
Mendelevium is a synthetic chemical element; it has symbol Md ( formerly Mv) and atomic number 101. A metallic radioactive transuranium element in the actinide series, it is the first element by atomic number that currently cannot be produced ...
onward was produced in fusion-evaporation reactions, culminating in the discovery of the heaviest known element oganesson
Oganesson is a synthetic element, synthetic chemical element; it has Chemical symbol, symbol Og and atomic number 118. It was first synthesized in 2002 at the Joint Institute for Nuclear Research (JINR) in Dubna, near Moscow, Russia, by a joint ...
in 2002 and most recently tennessine
Tennessine is a synthetic element; it has Chemical symbol, symbol Ts and atomic number 117. It has the second-highest atomic number and joint-highest atomic mass of all known elements and is the penultimate element of the Period 7 element, 7th ...
in 2010. These reactions approached the limit of current technology; for example, the synthesis of tennessine required 22 milligrams of 249Bk and an intense 48Ca beam for six months. The intensity of beams in superheavy element research cannot exceed 1012 projectiles per second without damaging the target and detector, and producing larger quantities of increasingly rare and unstable actinide
The actinide () or actinoid () series encompasses at least the 14 metallic chemical elements in the 5f series, with atomic numbers from 89 to 102, actinium through nobelium. Number 103, lawrencium, is also generally included despite being part ...
targets is impractical.
Consequently, future experiments must be done at facilities such as the superheavy element factory (SHE-factory) at the Joint Institute for Nuclear Research
The Joint Institute for Nuclear Research (JINR, ), in Dubna, Moscow Oblast (110 km north of Moscow), Russia, is an international research center for nuclear sciences, with 5,500 staff members including 1,200 researchers holding over 1,000 ...
(JINR) or RIKEN
is a national scientific research institute in Japan. Founded in 1917, it now has about 3,000 scientists on seven campuses across Japan, including the main site at Wakō, Saitama, Wakō, Saitama Prefecture, on the outskirts of Tokyo. Riken is a ...
, which will allow experiments to run for longer stretches of time with increased detection capabilities and enable otherwise inaccessible reactions.
It is possible that fusion-evaporation reactions will not be suitable for the discovery of unbibium or heavier elements. Various models predict increasingly short alpha
Alpha (uppercase , lowercase ) is the first letter of the Greek alphabet. In the system of Greek numerals, it has a value of one. Alpha is derived from the Phoenician letter ''aleph'' , whose name comes from the West Semitic word for ' ...
and spontaneous fission
Spontaneous fission (SF) is a form of radioactive decay in which a heavy atomic nucleus splits into two or more lighter nuclei. In contrast to induced fission, there is no inciting particle to trigger the decay; it is a purely probabilistic proc ...
half-lives for isotopes with ''Z'' = 122 and ''N'' ~ 180 on the order of microseconds or less, rendering detection nearly impossible with current equipment. The increasing dominance of spontaneous fission also may sever possible ties to known nuclei of livermorium or oganesson and make identification and confirmation more difficult; a similar problem occurred in the road to confirmation of the decay chain of 294Og which has no anchor to known nuclei. For these reasons, other methods of production may need to be researched such as multi-nucleon transfer reactions capable of populating longer-lived nuclei. A similar switch in experimental technique occurred when hot fusion using 48Ca projectiles was used instead of cold fusion (in which cross sections decrease rapidly with increasing atomic number) to populate elements with ''Z'' > 113.
Nevertheless, several fusion-evaporation reactions leading to unbibium have been proposed in addition to those already tried unsuccessfully, though no institution has immediate plans to make synthesis attempts, instead focusing first on elements 119, 120, and possibly 121. Because cross sections increase with asymmetry of the reaction, a chromium
Chromium is a chemical element; it has Symbol (chemistry), symbol Cr and atomic number 24. It is the first element in Group 6 element, group 6. It is a steely-grey, Luster (mineralogy), lustrous, hard, and brittle transition metal.
Chromium ...
beam would be most favorable in combination with a californium
Californium is a synthetic chemical element; it has symbol Cf and atomic number 98. It was first synthesized in 1950 at Lawrence Berkeley National Laboratory (then the University of California Radiation Laboratory) by bombarding curium with al ...
target, especially if the predicted closed neutron shell at ''N'' = 184 could be reached in more neutron-rich products and confer additional stability. In particular, the reaction between and would generate the compound nucleus and reach the shell at ''N'' = 184, though the analogous reaction with a target is believed to be more feasible because of the presence of unwanted fission product
Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the releas ...
s from and difficulty in accumulating the required amount of target material. One possible synthesis of unbibium could occur as follows:
: + → + 3
Should this reaction be successful and alpha decay remain dominant over spontaneous fission, the resultant 300Ubb would decay through 296Ubn which may be populated in cross-bombardment between 249Cf and 50Ti. Although this reaction is one of the most promising options for the synthesis of unbibium in the near future, the maximum cross section is predicted to be 3 fb, one order of magnitude lower than the lowest measured cross section in a successful reaction. The more symmetrical reactions 244Pu + 64Ni and 248Cm + 58Fe have also been proposed and may produce more neutron-rich isotopes. With increasing atomic number, one must also be aware of decreasing fission barrier
In nuclear physics and nuclear chemistry, the fission barrier is the activation energy required for a nucleus of an atom to undergo fission. This barrier may also be defined as the minimum amount of energy required to deform the nucleus to the ...
heights, resulting in lower survival probability of compound nuclei, especially above the predicted magic numbers at ''Z'' = 126 and ''N'' = 184.
Predicted properties
Nuclear stability and isotopes
plutonium
Plutonium is a chemical element; it has symbol Pu and atomic number 94. It is a silvery-gray actinide metal that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhibits six allotropes and four ...
, the heaviest primordial element
In geochemistry, geophysics and nuclear physics, primordial nuclides, also known as primordial isotopes, are nuclides found on Earth that have existed in their current form since before Earth was formed. Primordial nuclides were present in the ...
, so that all isotopes with an atomic number above 101
101 may refer to:
*101 (number), the number
* AD 101, a year in the 2nd century AD
* 101 BC, a year in the 2nd century BC
It may also refer to:
Entertainment
* ''101'' (album), a live album and documentary by Depeche Mode
* "101" (song), a 19 ...
decay radioactively with a half-life Half-life is a mathematical and scientific description of exponential or gradual decay.
Half-life, half life or halflife may also refer to:
Film
* Half-Life (film), ''Half-Life'' (film), a 2008 independent film by Jennifer Phang
* ''Half Life: ...
under a day. No elements with atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of its atomic nucleus. For ordinary nuclei composed of protons and neutrons, this is equal to the proton number (''n''p) or the number of pro ...
s above 82 (after lead
Lead () is a chemical element; it has Chemical symbol, symbol Pb (from Latin ) and atomic number 82. It is a Heavy metal (elements), heavy metal that is density, denser than most common materials. Lead is Mohs scale, soft and Ductility, malleabl ...
) have stable isotopes. Nevertheless, because of reasons not very well understood yet, there is a slight increased nuclear stability around atomic numbers 110
110 may refer to:
*110 (number), natural number
*AD 110, a year
*110 BC, a year
*110 film, a cartridge-based film format used in still photography
* 110 (MBTA bus), Massachusetts Bay Transportation Authority bus route
*110 (song), 2019 song by Cap ...
–114 114 may refer to:
*114 (number)
*AD 114
*114 BC
*114 (1st London) Army Engineer Regiment, Royal Engineers, an English military unit
*114 (Antrim Artillery) Field Squadron, Royal Engineers, a Northern Irish military unit
*114 (MBTA bus)
*114 (New Je ...
, which leads to the appearance of what is known in nuclear physics as the "island of stability
In nuclear physics, the island of stability is a predicted set of isotopes of superheavy elements that may have considerably longer half-lives than known isotopes of these elements. It is predicted to appear as an "island" in the chart of nuclid ...
". This concept, proposed by University of California
The University of California (UC) is a public university, public Land-grant university, land-grant research university, research university system in the U.S. state of California. Headquartered in Oakland, California, Oakland, the system is co ...
professor Glenn Seaborg
Glenn Theodore Seaborg ( ; April 19, 1912February 25, 1999) was an American chemist whose involvement in the synthesis, discovery and investigation of ten transuranium elements earned him a share of the 1951 Nobel Prize in Chemistry. His work i ...
, explains why superheavy element
Superheavy elements, also known as transactinide elements, transactinides, or super-heavy elements, or superheavies for short, are the chemical elements with atomic number greater than 104. The superheavy elements are those beyond the actinides in ...
s last longer than predicted.
In this region of the periodic table, ''N'' = 184 has been suggested as a closed neutron shell, and various atomic numbers have been proposed as closed proton shells, such as ''Z'' = 114, 120, 122, 124, and 126. The island of stability would be characterized by longer half-lives of nuclei located near these magic numbers, though the extent of stabilizing effects is uncertain due to predictions of weakening of the proton shell closures and possible loss of double magicity. More recent research predicts the island of stability to instead be centered at beta-stable copernicium
Copernicium is a synthetic chemical element; it has symbol Cn and atomic number 112. Its known isotopes are extremely radioactive, and have only been created in a laboratory. The most stable known isotope, copernicium-285, has a half-life of ap ...
isotopes 291Cn and 293Cn, which would place unbibium well above the island and result in short half-lives regardless of shell effects. The increased stability of elements 112–118 has also been attributed to the oblate
In Christianity (specifically the Roman Catholic, Orthodox, Lutheran, Anglican and Methodist traditions), an oblate is a person associated with a Benedictine monastery or convent who is specifically dedicated to God and service.
Oblates are i ...
shape of such nuclei and resistance to spontaneous fission. The same model also proposes 306Ubb as the next spherical doubly magic nucleus, thus defining the true island of stability for spherical nuclei.
proton emission
Proton emission (also known as proton radioactivity) is a rare type of radioactive decay in which a proton is ejected from a atomic nucleus, nucleus. Proton emission can occur from high-lying excited states in a nucleus following a beta decay ...
, 298–314Ubb will have alpha half-lives on the order of microseconds, and those heavier than 314Ubb will predominantly decay by spontaneous fission with short half-lives. For the lighter alpha emitters that may be populated in fusion-evaporation reactions, some long decay chains leading down to known or reachable isotopes of lighter elements are predicted. Additionally, the isotopes 308–310Ubb are predicted to have half-lives under 1 microsecond, too short for detection as a result of significantly lower binding energy
In physics and chemistry, binding energy is the smallest amount of energy required to remove a particle from a system of particles or to disassemble a system of particles into individual parts. In the former meaning the term is predominantly use ...
for neutron numbers immediately above the ''N'' = 184 shell closure. Alternatively, a second island of stability with total half-lives of approximately 1 second may exist around ''Z'' ~ 124 and ''N'' ~ 198, though these nuclei will be difficult or impossible to reach using current experimental techniques. However, these predictions are strongly dependent on the chosen nuclear mass models, and it is unknown which isotopes of unbibium will be most stable. Regardless, these nuclei will be hard to synthesize as no combination of obtainable target and projectile can provide enough neutrons in the compound nucleus. Even for nuclei reachable in fusion reactions, spontaneous fission and possibly also cluster decay
Cluster decay, also named heavy particle radioactivity, heavy ion radioactivity or heavy cluster decay," is a rare type of nuclear decay in which an atomic nucleus emits a small "cluster" of neutrons and protons, more than in an alpha particle, ...
might have significant branches, posing another hurdle to identification of superheavy elements as they are normally identified by their successive alpha decays.
Chemical
Unbibium is predicted to be similar in chemistry tocerium
Cerium is a chemical element; it has Chemical symbol, symbol Ce and atomic number 58. It is a hardness, soft, ductile, and silvery-white metal that tarnishes when exposed to air. Cerium is the second element in the lanthanide series, and while it ...
and thorium, which likewise have four valence electrons above a noble gas core, although it may be more reactive. Additionally, unbibium is predicted to belong to a new block of valence g-electron atoms, although the 5g orbital is not expected to start filling until about element 125. The predicted ground-state electron configuration of unbibium is either [ Og] 7d1 8s2 8p1 or 8s2 8p2, in contrast to the expected [ Og] 5g2 8s2 in which the 5g orbital starts filling at element 121. (The ds2p and s2p2 configurations are expected to be only separated by about 0.02 eV.) In the superactinides, relativistic effects might cause a breakdown of the Aufbau principle
In atomic physics and quantum chemistry, the Aufbau principle (, from ), also called the Aufbau rule, states that in the ground state of an atom or ion, electrons first fill Electron shell#Subshells, subshells of the lowest available energy, the ...
and create overlapping of the 5g, 6f, 7d and 8p orbitals; experiments on the chemistry of copernicium
Copernicium is a synthetic chemical element; it has symbol Cn and atomic number 112. Its known isotopes are extremely radioactive, and have only been created in a laboratory. The most stable known isotope, copernicium-285, has a half-life of ap ...
and flerovium
Flerovium is a synthetic chemical element; it has symbol Fl and atomic number 114. It is an extremely radioactive, superheavy element, named after the Flerov Laboratory of Nuclear Reactions of the Joint Institute for Nuclear Research in Du ...
provide strong indications of the increasing role of relativistic effects. As such, the chemistry of elements following unbibium becomes more difficult to predict.
Unbibium would most likely form a dioxide, Ubb O2, and tetrahalides, such as Ubb F4 and Ubb Cl4. The main oxidation state is predicted to be +4, similar to cerium and thorium. A first ionization energy of 5.651 eV and second ionization energy of 11.332 eV are predicted for unbibium; this and other calculated ionization energies are lower than the analogous values for thorium, suggesting that unbibium will be more reactive than thorium.
Notes
References
Bibliography
* * * * * *External links
Chemistry-Blog: Independent analysis of Marinov's 122 claim
{{Extended periodic table (by Fricke, 32 columns, compact)
122 122 may refer to:
* 122 (number), a natural number
* AD 122, a year in the 2nd century AD
* 122 BC, a year in the 2nd century BC
* ''122'' (film), a 2019 Egyptian psychological horror film
*" One Twenty Two", a 2022 single by the American rock band ...