|
Ytterbium(III) Oxalate
Ytterbium(III) oxalate is the oxalate of ytterbium, with the chemical formula Yb2(C2O4)3. Preparation Ytterbium(III) oxalate hydrate can be prepared by reacting an aqueous solution of ytterbium(III) chloride and a benzene solution of dimethyl oxalate Dimethyl oxalate is an organic compound with the formula or . It is the dimethyl ester of oxalic acid. Dimethyl oxalate is a colorless or white solid that is soluble in water. Production Dimethyl oxalate can be obtained by esterification of oxa .... Properties Ytterbium(III) oxalate pentahydrate is decomposed by heat to obtain the dihydrate, which is further heated to obtain ytterbium(III) oxide. It reacts with acids to obtain H b(C2O4)26H2O.Moebius, R.; Matthes, F. The exchange of oxalate ions for chloride ions of the oxalate hydrates of the rare earths and yttrium. ''Zeitschrift fuer Chemie'', 1964. 4 (6): 234-235. ISSN: 0044-2402. References {{Oxalates Ytterbium(III) compounds Oxalates ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cerium(III) Oxalate
Cerium(III) oxalate (cerous oxalate) is the Inorganic compound, inorganic cerium Salt (chemistry), salt of oxalic acid. It is a white crystalline solid with the chemical formula of Ce2(C2O4)3. It can be obtained by the reaction of oxalic acid with cerium(III) chloride. Uses Cerium(III) oxalate is used as an antiemetic. It has been identified as part of the invisible ink that was used by Stasi operatives during the Cold War. Toxicity Cerium(III) oxalate irritation, irritates human skin, skin and mucous membranes, and is a strong irritant to human eye, eyes. If it gets into the eyes, there is a danger of severe eye injury. Cerium salts increase the blood coagulation rate, and exposure to cerium salts can cause sensitivity to heat. Oxalates are corrosive to tissue and are powerful irritants. They have a caustic effect on the linings of the digestive tracts and can cause kidney damage. References {{Oxalates Cerium(III) compounds Oxalates Antiemetics ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thulium(III) Oxalate
Thulium(III) oxalate is the oxalate of thulium with the chemical formula Tm2(C2O4)3. Its hydrate can be prepared by reacting an aqueous solution of thulium(III) chloride and a benzene solution of dimethyl oxalate. Its pentahydrate is decomposed by heat to obtain the dihydrate, which is further heated to obtain thulium(III) oxide. It reacts with hydrochloric acid Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungency, pungent smell. It is classified as a acid strength, strong acid. It is ... to obtain H m(C2O4)2�6H2O.Moebius, R.; Matthes, F. The exchange of oxalate ions for chloride ions of the oxalate hydrates of the rare earths and yttrium. ''Zeitschrift fuer Chemie'', 1964. 4 (6): 234-235. ISSN: 0044-2402. References {{Oxalates Thulium compounds Oxalates ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
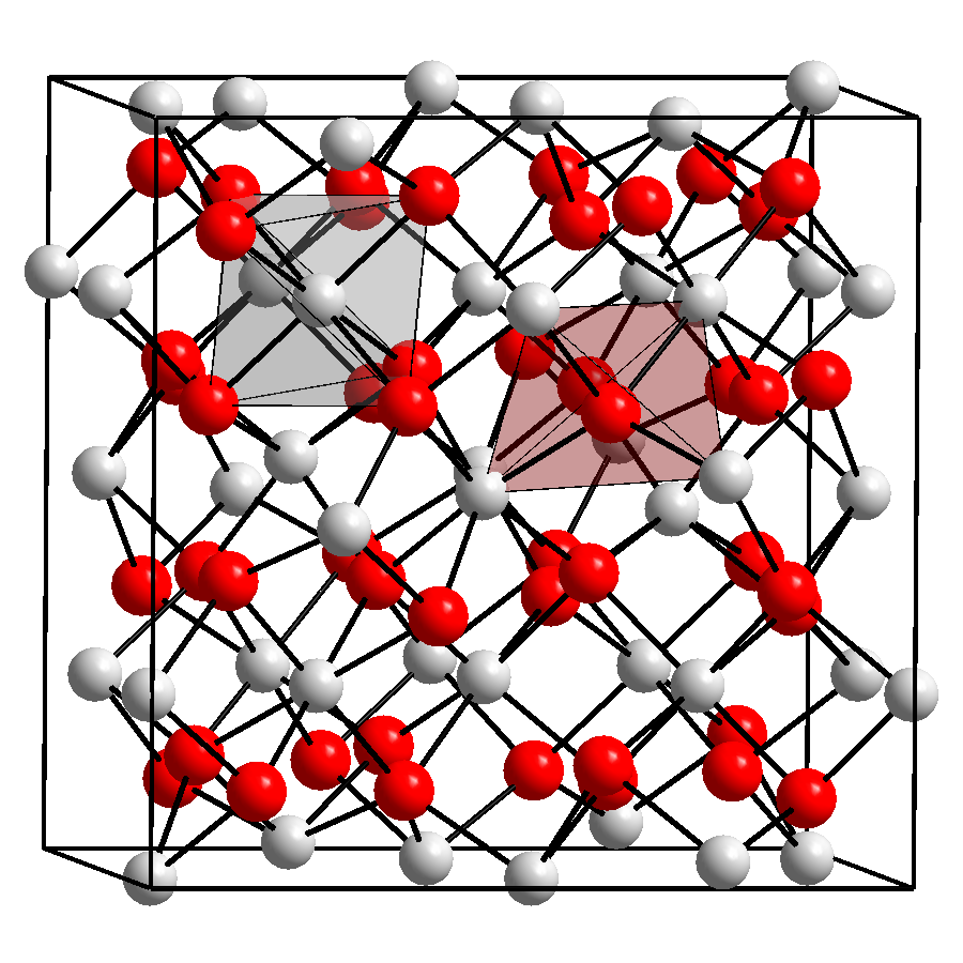
Ytterbium(III) Oxide
Ytterbium(III) oxide is the chemical compound with the formula Yb2O3. It is one of the more commonly encountered compounds of ytterbium. It occurs naturally in trace amounts in the mineral gadolinite. It was first isolated from this in 1878 by Jean Charles Galissard de Marignac. Preparation Ytterbium(III) oxide can be obtained by directly reacting ytterbium with oxygen: : It can also be obtained by the thermal decomposition of ytterbium carbonate or ytterbium oxalate at temperatures around 700 °C: : : Properties Chemical Ytterbium(III) oxide is a white powder. It reacts with carbon tetrachloride or hot hydrochloric acid to form ytterbium(III) chloride: : : Physical Like the other trivalent oxides of the heavier lanthanides, ytterbium(III) oxide has the "rare-earth C-type sesquioxide" structure which is related to the fluorite structure with one quarter of the anions removed, leading to ytterbium atoms in two different six coordinate (non-octahedral) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethyl Oxalate
Dimethyl oxalate is an organic compound with the formula or . It is the dimethyl ester of oxalic acid. Dimethyl oxalate is a colorless or white solid that is soluble in water. Production Dimethyl oxalate can be obtained by esterification of oxalic acid with methanol using sulfuric acid as a catalyst: :\rm 2\ CH_3OH + (CO_2H)_2\ \xrightarrow\ (CO_2CH_3)_2 + 2\ H_2O Oxidative carbonylation route The preparation by oxidative carbonylation has attracted interest because it requires only C1 precursors: :\rm 4 \ CH_3OH + 4 \ CO + O_2 \xrightarrow\ 2 \ (CO_2CH_3)_2 + 2 \ H_2O The reaction is catalyzed by Pd2+.E. Amadio''Oxidative Carbonylation of Alkanols Catalyzed by Pd(II)-Phosphine Complexes'' PhD Thesis, Ca'Foscari University Venice, 2009 The synthesis gas is mostly obtained from coal or biomass. The oxidation proceeds via dinitrogen trioxide, which is formed according to (1) of nitrogen monoxide and oxygen and then reacts according to (2) with methanol forming methyl nitrite: ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of petroleum and is one of the elementary petrochemicals. Due to the cyclic continuous pi bonds between the carbon atoms, benzene is classed as an aromatic hydrocarbon. Benzene is a colorless and highly Combustibility and flammability, flammable liquid with a sweet smell, and is partially responsible for the aroma of gasoline. It is used primarily as a Precursor (chemistry), precursor to the manufacture of chemicals with more complex structures, such as ethylbenzene and cumene, of which billions of kilograms are produced annually. Although benzene is a major Chemical industry, industrial che ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ytterbium(III) Chloride
Ytterbium(III) chloride ( Yb Cl3) is an inorganic compound. It was first synthesized by Jan Hoogschagen in 1946. It is a paramagnetic Lewis acid, like many of the lanthanide chlorides. This gives rise to pseudocontact shifted NMR spectra, akin to NMR shift reagents. It reacts with NiCl2 to form a very effective catalyst for the reductive dehalogenation of aryl halides. Chemical properties The valence electron configuration of Yb+3 (from YbCl3) is 4''f''135''s''25''p''6, which has crucial implications for the chemical behaviour of Yb+3. Also, the size of Yb+3 governs its catalytic behaviour and biological applications. For example, while both Ce+3 and Yb+3 have a single unpaired ''f'' electron, Ce+3 is much larger than Yb+3 because lanthanides become much smaller with increasing effective nuclear charge as a consequence of the ''f'' electrons not being as well shielded as ''d'' electrons. This behavior is known as the lanthanide contraction. The small size of Yb+3 produces fast ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ytterbium
Ytterbium is a chemical element; it has symbol Yb and atomic number 70. It is a metal, the fourteenth and penultimate element in the lanthanide series, which is the basis of the relative stability of its +2 oxidation state. Like the other lanthanides, its most common oxidation state is +3, as in its oxide, halides, and other compounds. In aqueous solution, like compounds of other late lanthanides, soluble ytterbium compounds form complexes with nine water molecules. Because of its closed-shell electron configuration, its density, melting point and boiling point are much lower than those of most other lanthanides. In 1878, Swiss chemist Jean Charles Galissard de Marignac separated from the rare earth "erbia", another independent component, which he called " ytterbia", for Ytterby, the village in Sweden near where he found the new component of erbium. He suspected that ytterbia was a compound of a new element that he called "ytterbium". Four elements were named after the village, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxalate
Oxalate (systematic IUPAC name: ethanedioate) is an anion with the chemical formula . This dianion is colorless. It occurs naturally, including in some foods. It forms a variety of salts, for example sodium oxalate (), and several esters such as dimethyl oxalate (). It is a conjugate base of oxalic acid. At neutral pH in aqueous solution, oxalic acid converts completely to oxalate. Relationship to oxalic acid The dissociation of protons from oxalic acid proceeds in a determined order; as for other polyprotic acids, loss of a single proton results in the monovalent hydrogenoxalate anion . A salt with this anion is sometimes called an acid oxalate, monobasic oxalate, or hydrogen oxalate. The equilibrium constant ( ''K''a) for loss of the first proton is ( p''K''a = 1.27). The loss of the second proton, which yields the oxalate ion, has an equilibrium constant of (p''K''a = 4.28). These values imply, in solutions with neutral pH, no oxalic acid and only t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Terbium(III) Oxalate
Terbium(III) oxalate is the oxalate of terbium with the chemical formula Tb2(C2O4)3. Its decahydrate can be obtained by reacting terbium(III) chloride and oxalic acid in an aqueous solution. Its decahydrate gradually loses water when heated and becomes anhydrous. Continued heating obtains terbium(III,IV) oxide. It decomposes in isolation from air to form terbium(III) oxide. The decomposed gas products are carbon monoxide and carbon dioxide. It reacts with hydrochloric acid Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungency, pungent smell. It is classified as a acid strength, strong acid. It is ... to obtain H b(C2O4)2�6H2O.Moebius, R.; Matthes, F. The exchange of oxalate ions for chloride ions of the oxalate hydrates of the rare earths and yttrium. ''Zeitschrift fuer Chemie'', 1964. 4 (6): 234-235. ISSN: 0044-2402. References {{Oxalates Terbium comp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Europium(III) Oxalate
Europium(III) oxalate (Eu2(C2O4)3) is a chemical compound of europium and oxalic acid. There are different hydrates including the decahydrate, hexahydrate and tetrahydrate. Europium(II) oxalate is also known. Preparation An excess of oxalate is added to a hot solution of Eu3+ cations. The resulting precipitate of Eu2(C2O4)3 ⋅ 10H2O is dried in a desiccator. Properties Europium(III) oxide (Eu2O3) can be prepared by calcining europium(III) oxalate. The dehydration of Eu2(C2O4)3 · 10H2O occurs below 200 °C: : The decomposition of this compound takes place in two stages, the first at 350 °C and the second at about 620 °C. : In the Mössbauer spectrum, Eu2(C2O4)3 · 10H2O shows an isomer shift of +0,26 mm/s with a line width of 2,38 mm/s, in reference to EuF3. The Debye temperature of Eu2(C2O4)3 is 166±15 K. Eu2(C2O4)3 · 10H2O crystallizes monoclinically in the space group of ''P21/c'' (space g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Samarium(III) Oxalate
Samarium(III) oxalate is an inorganic compound, a salt of samarium and oxalic acid with the formula Sm2(C2O4)3. The compound does not dissolve in water, forms a crystalline hydrate with yellow crystals. Synthesis Precipitation In meteorology, precipitation is any product of the condensation of atmospheric water vapor that falls from clouds due to gravitational pull. The main forms of precipitation include drizzle, rain, rain and snow mixed ("sleet" in Commonwe ... of soluble samarium salts with oxalic acid: ::\mathsf Also a reaction of samarium nitrate and oxalic acid in an aqueous solution: ::\mathsf Physical properties Samarium(III) oxalate forms a crystalline hydrate of the composition Sm2(C2O4)3 • 10H2O with yellow crystals. Chemical properties Decomposes on heating: ::\mathsf Crystalline hydrate Sm2(C2O4)3 • 10H2O decomposes stepwise. References {{Oxalates Oxalates Samarium(III) compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Promethium(III) Oxalate
Promethium(III) oxalate is an oxalate of promethium, with the chemical formula Pm2(C2O4)3. Its decahydrate crystallizes in the monoclinic crystal system with space group ''P''21/''m''. Promethium(III) oxalate trihydrate can decompose into stable basic carbonate Pm2O2CO3, and generate promethium(III) oxide Promethium(III) oxide is a compound with the formula Pm2O3. It is the most common form of promethium. Crystal structure Promethium oxide exists in three major crystalline forms: *a, b and c are lattice parameters, Z is the number of formula ... at higher temperatures. In all lanthanide oxalates, promethium(III) oxalate has the lowest solubility. References {{Oxalates Promethium compounds Oxalates ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |