|
Viral Nucleoprotein
Viral nucleoproteins (NPs) are essential RNA-binding protein, RNA-binding proteins encoded by many Virus, viruses, especially Negative-strand RNA virus, negative-sense single-stranded RNA (–ssRNA) viruses. They play crucial roles in Encapsulating peritoneal sclerosis, encapsulating viral RNA, facilitating Viral replication, genome replication and Transcription (biology), transcription, organizing viral Nucleoprotein, ribonucleoprotein (vRNP) complexes, and Immune system, evading host immunity. Structure and function Key functions of viral NPs include: * RNA Encapsulation: NPs coat the viral genome in a sequence-independent manner, protecting it from Nuclease, nucleases and Pattern recognition receptor, host pattern recognition receptors such as RIG-I and MDA5. * RNP Assembly: NP-RNA complexes serve as templates for viral RNA synthesis by the RNA-dependent RNA polymerase (RdRp). * Regulation of Replication: NP levels help determine the balance between Transcription (biology), tr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
RNA-binding Protein
RNA-binding proteins (often abbreviated as RBPs) are proteins that bind to the double or single stranded RNA in cell (biology), cells and participate in forming ribonucleoprotein complexes. RBPs contain various structural motifs, such as RNA recognition motif (RRM), dsRNA binding domain, zinc finger and others. They are cytoplasmic and cell nucleus, nuclear proteins. However, since most mature RNA is exported from the nucleus relatively quickly, most RBPs in the nucleus exist as complexes of protein and Precursor mRNA, pre-mRNA called heterogeneous ribonucleoprotein particles (hnRNPs). RBPs have crucial roles in various cellular processes such as: cellular function, transport and localization. They especially play a major role in post-transcriptional control of RNAs, such as: RNA splicing, splicing, polyadenylation, mRNA stabilization, mRNA Subcellular localization, localization and Translation (biology), translation. Eukaryote, Eukaryotic cells express diverse RBPs with unique RN ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Viral Matrix Protein
Viral matrix proteins are structural proteins linking the viral envelope with the virus core. They play a crucial role in virus assembly, and interact with the RNP complex as well as with the viral membrane. They are found in many enveloped viruses including paramyxoviruses, orthomyxoviruses, herpesviruses, retroviruses, filoviruses and other groups. An example is the M1 protein of the influenza virus, showing affinity to the glycoproteins inserted in the host cell membrane on one side and affinity for the RNP complex molecules on the other side, which allows formation at the membrane of a complex made of the viral ribonucleoprotein at the inner side indirectly connected to the viral glycoproteins protruding from the membrane. This assembly complex will now bud In botany, a bud is an undeveloped or Plant embryogenesis, embryonic Shoot (botany), shoot and normally occurs in the axil of a leaf or at the tip of a Plant stem, stem. Once formed, a bud may remain for some ti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
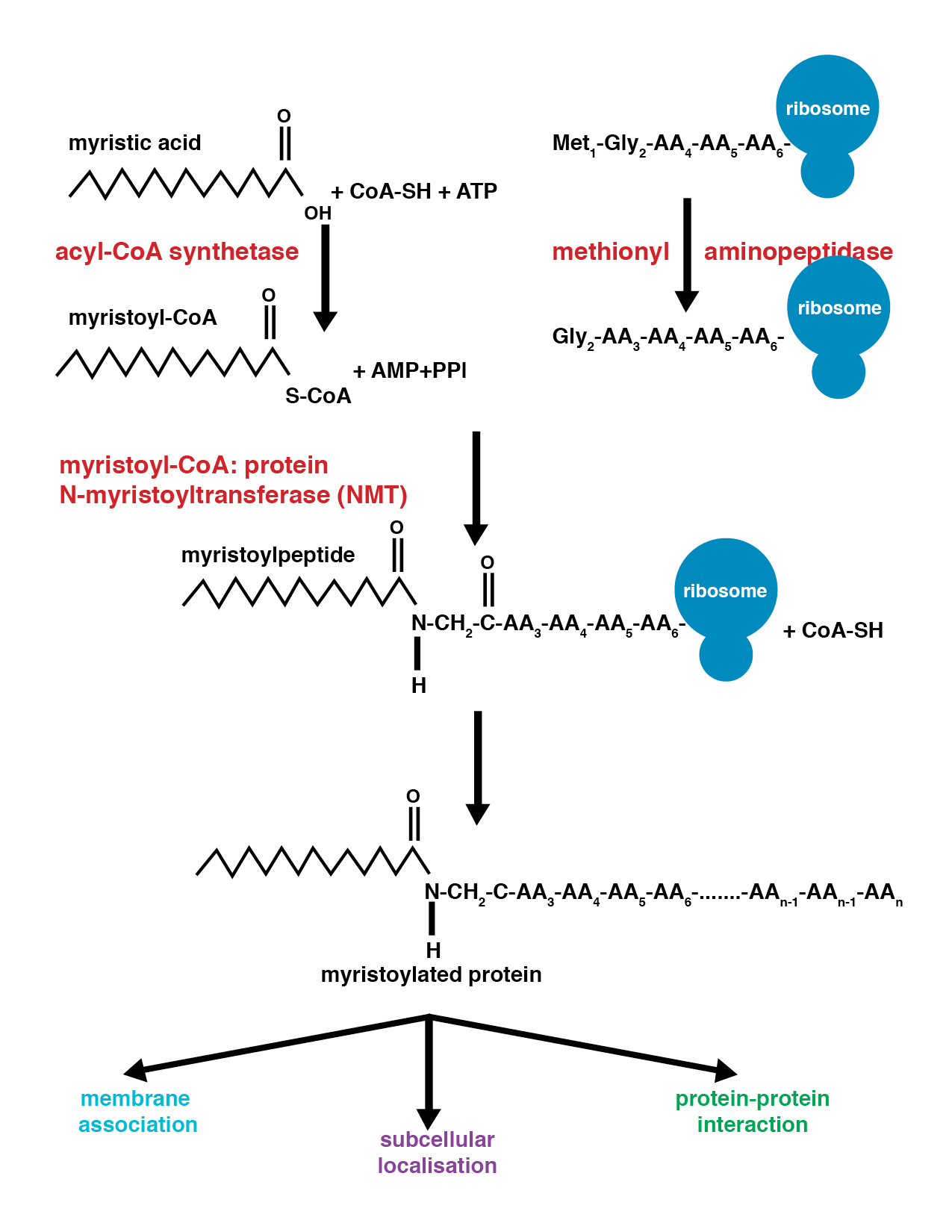
Myristoylation
Myristoylation is a lipidation modification where a myristoyl group, derived from myristic acid, is covalently attached by an amide bond to the alpha-amino group of an ''N''-terminal glycine residue. Myristic acid is a 14-carbon saturated fatty acid (14:0) with the systematic name of ''n''-tetradecanoic acid. This modification can be added either co-translationally or post-translationally. ''N''-myristoyltransferase (NMT) catalyzes the myristic acid addition reaction in the cytoplasm of cells. This lipidation event is the most common type of fatty acylation and is present in many organisms, including animals, plants, fungi, protozoans and viruses. Myristoylation allows for weak protein–protein and protein–lipid interactions and plays an essential role in membrane targeting, protein–protein interactions and functions widely in a variety of signal transduction pathways. Discovery In 1982, Koiti Titani's lab identified an "''N''-terminal blocking group" on the catal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lymphocytic Choriomeningitis
Lymphocytic choriomeningitis (LCM) is a rodent-borne viral infectious disease that presents as aseptic meningitis, encephalitis or meningoencephalitis. Its causative agent is lymphocytic choriomeningitis virus (LCMV), a member of the family '' Arenaviridae''. The name was coined by Charles Armstrong in 1934. Lymphocytic choriomeningitis (LCM) is "a viral infection of the membranes surrounding the brain and spinal cord and of the cerebrospinal fluid". Lasker, Jill S. "Lymphocytic choriomeningitis". The name is based on the tendency of an individual to have abnormally high levels of lymphocytes during infection. Choriomeningitis is "cerebral meningitis in which there is marked cellular infiltration of the meninges, often with a lymphocytic infiltration of the choroid plexuses". Signs and symptoms LCMV infection manifests itself in a wide range of clinical symptoms, and may even be asymptomatic for immunocompetent individuals. CDC. "Information for Pet Owners: Reducing the Ri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lassa Virus
Lassa virus (LASV) is an Arenaviridae, arenavirus that causes Lassa hemorrhagic fever, a type of viral hemorrhagic fever (VHF), in humans and other primates. Lassa virus is an Emerging infectious disease, emerging virus and a select agent, requiring Biosafety level#Biosafety level 4, Biosafety Level 4-equivalent containment. It is endemic in West African countries, especially Sierra Leone, the Republic of Guinea, Nigeria, and Liberia, where the annual incidence of infection is between 300,000 and 500,000 cases, resulting in 5,000 deaths per year. As of 2012 discoveries within the Mano River region of west Africa have expanded the endemic zone between the two known Lassa endemic regions, indicating that LASV is more widely distributed throughout the tropical wooded savannah ecozone in west Africa. There are no approved vaccines against Lassa fever for use in humans. Discovery In 1969, missionary nurse Laura Wine fell ill with a mysterious disease she contracted from an obstetri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mammarenavirus
''Mammarenavirus'' is a genus of viruses in the family ''Arenaviridae''. The name is a portmanteau of mammal and the former name ''Arenavirus'', and differentiates it from the reptile-associated ''Reptarenavirus''. ''Arenavirus'' comes from the Latin (sand) for the sandy appearance of the virions. Taxonomy The genus contains the following species, listed by scientific name and followed by the exemplar virus of the species: * ''Mammarenavirus abaense'', Ābà-Miányáng virus * ''Mammarenavirus alashanense'', Alxa virus * ''Mammarenavirus allpahuayoense'', Allpahuayo virus * ''Mammarenavirus amapariense'', Amaparí virus * ''Mammarenavirus aporeense'', Aporé virus * ''Mammarenavirus batangense'', Bātáng virus * ''Mammarenavirus bearense'', Bear Canyon virus * ''Mammarenavirus beregaense'', Berega virus * ''Mammarenavirus bituense'', Bitu virus * ''Mammarenavirus brazilense'', Sabiá virus * ''Mammarenavirus caliense'', Pichindé virus * ''Mammarenavirus cameroonense'', Souri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SUMO Protein
In molecular biology, SUMO (Small Ubiquitin-like Modifier) proteins are a Protein family, family of small proteins that are covalent bond, covalently attached to and detached from other proteins in cell (biology), cells to modify their function. This process is called SUMOylation (pronounced soo-muh-lā-shun and sometimes written sumoylation). SUMOylation is a post-translational modification involved in various cellular processes, such as cell nucleus, nuclear-cytosolic transport, transcription (genetics), transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle. In human proteins, there are over 53,000 SUMO binding sites, making it a substantial component of fundamental biology. SUMO proteins are similar to ubiquitin and are considered members of the ubiquitin-like protein family. SUMOylation is directed by an Biochemical cascade, enzymatic cascade analogous to that involved in ubiquitination. In contrast to ubiquitin, SU ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Post-translational Modification
In molecular biology, post-translational modification (PTM) is the covalent process of changing proteins following protein biosynthesis. PTMs may involve enzymes or occur spontaneously. Proteins are created by ribosomes, which translation (biology), translate mRNA into polypeptide chains, which may then change to form the mature protein product. PTMs are important components in cell signal transduction, signalling, as for example when prohormones are converted to hormones. Post-translational modifications can occur on the amino acid side chains or at the protein's C-terminus, C- or N-terminus, N- termini. They can expand the chemical set of the 22 proteinogenic amino acid, amino acids by changing an existing functional group or adding a new one such as phosphate. Phosphorylation is highly effective for controlling the enzyme activity and is the most common change after translation. Many eukaryotic and prokaryotic proteins also have carbohydrate molecules attached to them in a pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
XPO1
Exportin 1 (XPO1), also known as chromosomal region maintenance 1 (CRM1), is a eukaryotic protein that mediates the nuclear export of various proteins and RNAs. History XPO1 (CRM1) originally was identified in the fission yeast ''Schizosaccharomyces pombe'' in a genetic screen, and investigators determined that it was involved in control of the chromosome structure. It was later shown to be the nuclear transport receptor for cargos with leucine-rich nuclear export signals ( NES). The structural details of the interaction of XPO1 with its cargos were revealed two decades after the gene was identified. Function XPO1 mediates NES-dependent protein transport. It exports several hundreds of different proteins from the nucleus. XPO1 is involved in the nuclear export of ribosomal subunits. XPO1 plays a role in export of various RNAs including U snRNAs, rRNAs (as a part of ribosomal subunits), and some mRNAs. Medical relevance XPO1 is involved in various viral infections. For e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
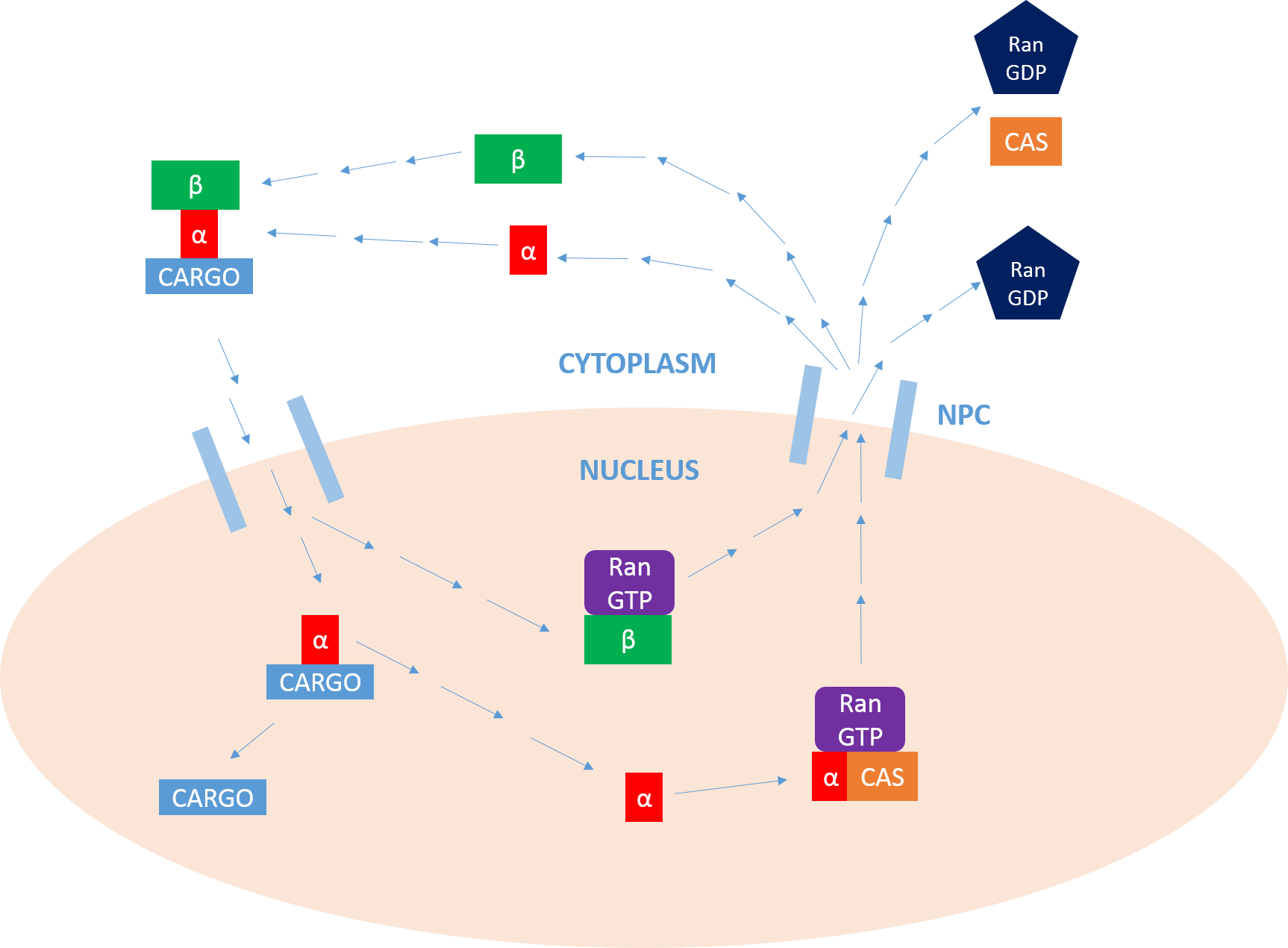
Importin
Importin is a type of karyopherin that transports protein molecules from the Eukaryotic Cell, cell's cytoplasm to the cell nucleus, nucleus. It does so by binding to specific recognition sequences, called nuclear localization sequences (NLS). Importin has two subunits, importin α and importin β. Members of the importin-β family can bind and transport cargo by themselves, or can form heterodimers with importin-α. As part of a heterodimer, importin-β mediates interactions with the nuclear pore, pore complex, while importin-α acts as an adaptor protein to bind the nuclear localization signal (NLS) on the cargo. The NLS-Importin α-Importin β protein trimer, trimer dissociates after binding to Ran (gene), Ran Guanosine triphosphate, GTP inside the Cell nucleus, nucleus, with the two importin proteins being recycled to the cytoplasm for further use. Discovery Importin can exist as either a heterodimer of importin-α/β or as a monomer of Importin-β. Importin-α was first isol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymerase
In biochemistry, a polymerase is an enzyme (Enzyme Commission number, EC 2.7.7.6/7/19/48/49) that synthesizes long chains of polymers or nucleic acids. DNA polymerase and RNA polymerase are used to assemble DNA and RNA molecules, respectively, by copying a DNA template strand using Base pair, base-pairing interactions or RNA by half ladder replication. A DNA polymerase from the thermophile, thermophilic bacterium, ''Thermus aquaticus'' (''Taq'') (Protein Data Bank, PDB]1BGX EC 2.7.7.7) is used in the polymerase chain reaction, an important technique of molecular biology. A polymerase may be template-dependent or template-independent. Polynucleotide adenylyltransferase, Poly-A-polymerase is an example of template independent polymerase. Terminal deoxynucleotidyl transferase also known to have template independent and template dependent activities. By function *DNA polymerase (DNA-directed DNA polymerase, DdDP) **Family A: DNA polymerase I; Pol POLG, γ, POLQ, θ, DNA polymer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |