|
Trichloroacetonitrile
Trichloroacetonitrile is an organic compound with the formula CCl3CN. It is a colourless liquid, although commercial samples often are brownish. It is used commercially as a precursor to the fungicide etridiazole. It is prepared by dehydration of trichloroacetamide. As a bifunctional compound, trichloroacetonitrile can react at both the trichloromethyl and the nitrile group. The Electrophilic aromatic directing groups, electron-withdrawing effect of the trichloromethyl group activates the nitrile group for nucleophilic additions. The high Reactivity (chemistry), reactivity makes trichloroacetonitrile a versatile reagent, but also causes its susceptibility towards hydrolysis. Synthesis The production of trichloroacetonitrile by dehydration of trichloroacetamide was first described in 1873 by L. Bisschopinck at the KU Leuven, Katholieke Universiteit Leuven. : Trichloroacetonitrile can be obtained by Chlorination reaction, chlorination of acetonitrile on a zinc, copper and alkali ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-containing compounds such as alkanes (e.g. methane ) and its derivatives are universally considered organic, but many others are sometimes considered inorganic, such as certain compounds of carbon with nitrogen and oxygen (e.g. cyanide ion , hydrogen cyanide , chloroformic acid , carbon dioxide , and carbonate ion ). Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, and even ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Activated Carbon
Activated carbon, also called activated charcoal, is a form of carbon commonly used to filter contaminants from water and air, among many other uses. It is processed (activated) to have small, low-volume pores that greatly increase the surface area available for ''adsorption '' or chemical reactions. (Adsorption, not to be confused with Absorption (chemistry), absorption, is a process where atoms or molecules adhere to a surface). The pores can be thought of as a microscopic "sponge" structure. Activation is analogous to making popcorn from dried corn kernels: popcorn is light, fluffy, and its kernels have a high surface-area-to-volume ratio. ''Activated'' is sometimes replaced by ''active''. Because it is so porous on a microscopic scale, one gram of activated carbon has a surface area of over , as determined by gas absorption and its porosity can run 10ML/day in terms of treated water per gram. Researchers at Cornell University synthesized an ultrahigh surface area activated ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
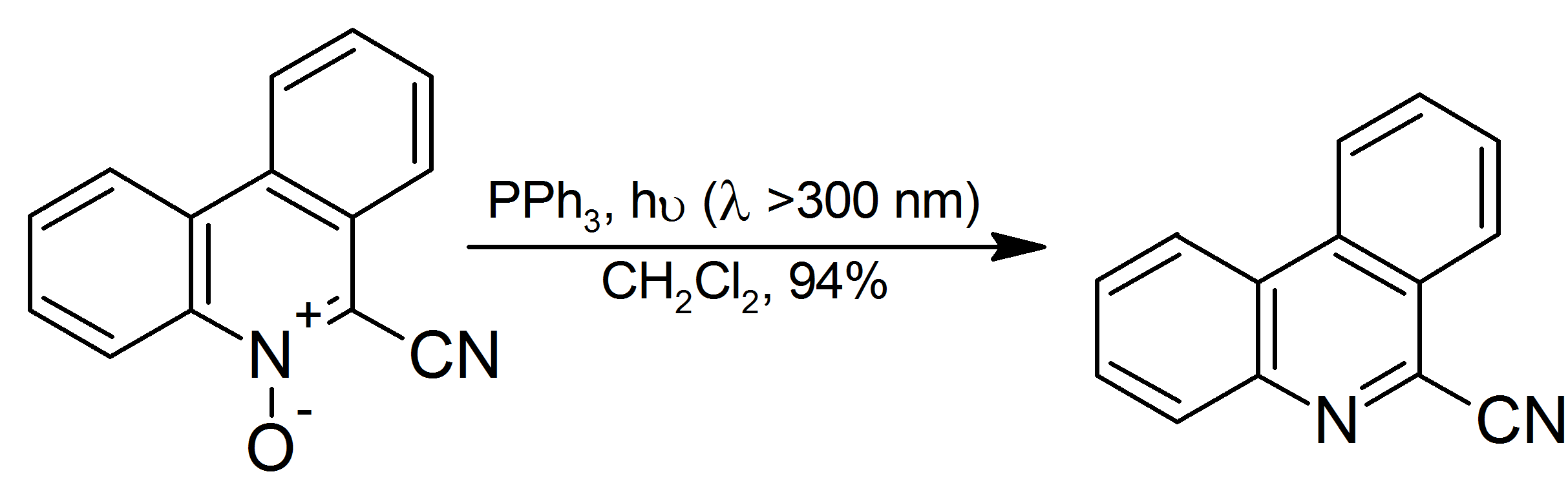
Triphenylphosphine
Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to P Ph3 or Ph3P. It is versatile compound that is widely used as a reagent in organic synthesis and as a ligand for transition metal complexes, including ones that serve as catalysts in organometallic chemistry. PPh3 exists as relatively air stable, colorless crystals at room temperature. It dissolves in non-polar organic solvents such as benzene and diethyl ether. Preparation and structure Triphenylphosphine can be prepared in the laboratory by treatment of phosphorus trichloride with phenylmagnesium bromide or phenyllithium. The industrial synthesis involves the reaction between phosphorus trichloride, chlorobenzene, and sodium: :PCl3 + 3 PhCl + 6 Na → PPh3 + 6 NaCl Triphenylphosphine crystallizes in triclinic and monoclinic modification. In both cases, the molecule adopts a pyramidal structure with propeller-like arrangement of the thre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orthocarbonic Acid
Orthocarbonic acid, carbon hydroxide, or methanetetrol is the name given to a hypothetical compound with the chemical formula or . Its molecular structure consists of a single carbon atom bonded to four hydroxyl groups. It would be therefore a fourfold Alcohol (chemistry), alcohol. In theory it could lose four protons to give the hypothetical oxocarbon anion orthocarbonate , and is therefore considered an oxoacid of carbon. Orthocarbonic acid is highly unstable. Calculations show that it decomposes into carbonic acid and water: : Orthocarbonic acid is one of the group of ''ortho acids'' that have the general structure of . The term ''ortho acid'' is also used to refer to the most hydroxylated acid in a set of oxoacids. Researchers predict that orthocarbonic acid is stable at high pressure; hence it may form in the interior of the ice giant planets Uranus and Neptune, where water and methane are common. Orthocarbonate anions By loss of one through four protons, orthocarbonic ac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkoxide
In chemistry, an alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They are written as , where R is the organyl substituent. Alkoxides are strong bases and, when R is not bulky, good nucleophiles and good ligands. Alkoxides, although generally not stable in protic solvents such as water, occur widely as intermediates in various reactions, including the Williamson ether synthesis.excerpt Transition metal alkoxides are widely used for coatings and as catalysts. Enolates are unsaturated alkoxides derived by deprotonation of a bond adjacent to a ketone or aldehyde. The nucleophilic center for simple alkoxides is located on the oxygen, whereas the nucleophilic site on enolates is delocalized onto both carbon and oxygen sites. Ynolates are also unsaturated alkoxides derived from acetylenic alcohols. Phenoxides are close relatives of the alkoxides, in which the alkyl group is replaced by a phe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CRC Handbook Of Chemistry And Physics
The ''CRC Handbook of Chemistry and Physics'' is a comprehensive one-volume reference resource for science research. First published in 1914, it is currently () in its 105th edition, published in 2024. It is known colloquially among chemists as the "Rubber Bible", as CRC originally stood for "Chemical Rubber Company". As late as the 1962–1963 edition (3604 pages), the ''Handbook'' contained myriad information for every branch of science and engineering. Sections in that edition include: Mathematics, Properties and Physical Constants, Chemical Tables, Properties of Matter, Heat, Hygrometric and Barometric Tables, Sound, Quantities and Units, and Miscellaneous. ''Mathematical Tables from Handbook of Chemistry and Physics'' was originally published as a supplement to the handbook up to the 9th edition (1952); afterwards, the 10th edition (1956) was published separately as '' CRC Standard Mathematical Tables''. Earlier editions included sections such as "Antidotes of Poisons", ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metre
The metre (or meter in US spelling; symbol: m) is the base unit of length in the International System of Units (SI). Since 2019, the metre has been defined as the length of the path travelled by light in vacuum during a time interval of of a second, where the second is defined by a hyperfine transition frequency of caesium. The metre was originally defined in 1791 by the French National Assembly as one ten-millionth of the distance from the equator to the North Pole along a great circle, so the Earth's polar circumference is approximately . In 1799, the metre was redefined in terms of a prototype metre bar. The bar used was changed in 1889, and in 1960 the metre was redefined in terms of a certain number of wavelengths of a certain emission line of krypton-86. The current definition was adopted in 1983 and modified slightly in 2002 to clarify that the metre is a measure of proper length. From 1983 until 2019, the metre was formally defined as the length of the pat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bond Length
In molecular geometry, bond length or bond distance is defined as the average distance between Atomic nucleus, nuclei of two chemical bond, bonded atoms in a molecule. It is a Transferability (chemistry), transferable property of a bond between atoms of fixed types, relatively independent of the rest of the molecule. Explanation Bond length is related to bond order: when more electrons participate in bond formation the bond is shorter. Bond length is also inversely related to bond strength and the bond dissociation energy: all other factors being equal, a stronger bond will be shorter. In a bond between two identical atoms, half the bond distance is equal to the covalent radius. Bond lengths are measured in the solid phase by means of X-ray diffraction, or approximated in the gas phase by microwave spectroscopy. A bond between a given pair of atoms may vary between different molecules. For example, the carbon to hydrogen bonds in methane are different from those in methyl chlori ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Humic Acid
Humic substances (HS) are colored relatively recalcitrant organic compounds naturally formed during long-term decomposition and transformation of biomass residues. The color of humic substances varies from bright yellow to light or dark brown leading to black. The term comes from humus, which in turn comes from the Latin word '' humus'', meaning "soil, earth". Humic substances represent the major part of organic matter in soil, peat, coal, and sediments, and are important components of dissolved natural organic matter (NOM) in lakes (especially dystrophic lakes), rivers, and sea water. Humic substances account for 50 – 90% of cation exchange capacity in soils. "Humic substances" is an umbrella term covering humic acid, fulvic acid and humin, which differ in solubility. By definition, humic acid (HA) is soluble in water at neutral and alkaline pH, but insoluble at acidic pH < 2. Fulvic acid (FA) is soluble in water at any pH. Humin is not soluble in water at any pH. This def ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Algae
Algae ( , ; : alga ) is an informal term for any organisms of a large and diverse group of photosynthesis, photosynthetic organisms that are not plants, and includes species from multiple distinct clades. Such organisms range from unicellular microalgae, such as cyanobacteria, ''Chlorella'', and diatoms, to multicellular macroalgae such as kelp or brown algae which may grow up to in length. Most algae are aquatic organisms and lack many of the distinct cell and tissue types, such as stomata, xylem, and phloem that are found in embryophyte, land plants. The largest and most complex marine algae are called seaweeds. In contrast, the most complex freshwater forms are the Charophyta, a Division (taxonomy), division of green algae which includes, for example, ''Spirogyra'' and stoneworts. Algae that are carried passively by water are plankton, specifically phytoplankton. Algae constitute a Polyphyly, polyphyletic group because they do not include a common ancestor, and although Eu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Evonik Degussa
{{Infobox company , name = Evonik Industries AG , logo = Logo Evonik 2020.svg , image = RellingHaus II, Essen.jpg , image_caption = Evonik's headquarters in Essen, Germany , type = Aktiengesellschaft , traded_as = {{Frankfurt Stock Exchange, EVK MDAX Component , industry = Specialty chemicals , key_people = {{plainlist , * Christian Kullmann {{small, (CEO, Chairman) * Bernd Tönjes (Chairman of the supervisory board) , products = Chemicals , revenue = {{Decrease €15.2 billion (2024){{cite web , url= https://www.evonik.com/en/investor-relations/Reporting.html#tabs-b118b10b7a-item-2d4ab0c2ad-tab , title=Evonik Financial Report 2024 , publisher=Evonik Industries AG , access-date=1 April 2025 , net_income = {{Decrease €222 million (2024) , num_employees = 31,930 (2024) , foundation = {{start date and age, df=yes, 2007 , location = Essen, Germany , homepage = www.evonik.c Evonik Industries AG is a publicly-listed German specialty Chemical substance, chemicals compa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |