|
Orthocarbonic Acid
Orthocarbonic acid, carbon hydroxide, or methanetetrol is the name given to a hypothetical compound with the chemical formula or . Its molecular structure consists of a single carbon atom bonded to four hydroxyl groups. It would be therefore a fourfold Alcohol (chemistry), alcohol. In theory it could lose four protons to give the hypothetical oxocarbon anion orthocarbonate , and is therefore considered an oxoacid of carbon. Orthocarbonic acid is highly unstable. Calculations show that it decomposes into carbonic acid and water: : Orthocarbonic acid is one of the group of ''ortho acids'' that have the general structure of . The term ''ortho acid'' is also used to refer to the most hydroxylated acid in a set of oxoacids. Researchers predict that orthocarbonic acid is stable at high pressure; hence it may form in the interior of the ice giant planets Uranus and Neptune, where water and methane are common. Orthocarbonate anions By loss of one through four protons, orthocarbonic ac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetramethoxymethane
Tetramethoxymethane is a chemical compound which is formally formed by complete methylation of the hypothetical orthocarbonic acid . Preparation The obvious synthetic route from the tetrahalomethanes does not yield the desired product, instead giving orthoformates and a halohydrin byproduct.R. H. De Wolfe, ''Carboxylic ortho acid derivatives: preparation and synthetic applications'', Organic Chemistry, Vol. 14, Academic Press, Inc. New York – London, 1970, . The original preparation of the tetramethoxymethane was therefore based on chloropicrin: : Because of the extreme toxicity of chloropicrin, other tetrasubstituted reactive methane derivatives were investigated as starting material for tetramethoxymethane. For example, trichloromethanesulfenyl chloride (also used as a chemical warfare agent and easily accessible from carbon disulfide and chlorine) was used:H. Tieckelmann, H. W. Post, ''The preparation of methyl, ethyl, propyl, and butyl orthocarbonates'', J. Org. Chem., 13 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orthonitrate
Orthonitrate is a tetrahedral anion of nitrogen with the formula . It was first identified in 1977 and is currently known in only two compounds, sodium orthonitrate (Na3NO4) and potassium orthonitrate (K3NO4). The corresponding oxoacid, orthonitric acid (H3NO4), is hypothetical and has never been observed. Sodium and potassium orthonitrate can be prepared by fusion of the nitrate and metal oxide under high temperatures and ideally high pressures (several GPa). : (300 °C for 3 days) The resulting orthonitrates are white solids which are extremely sensitive to moisture and CO2, decomposing within minutes to hydroxides, carbonates, and nitrates upon exposure to air. : : The orthonitrate ion is tetrahedral with N–O bond lengths of 139 pm, which is unexpectedly short, indicating that polar interactions are shortening the bond. This short bond length parallels that of hypervalent oxyanions containing third-row elements like and , for which pπ–dπ bonding was pre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ice Giant
An ice giant is a giant planet composed mainly of elements heavier than hydrogen and helium, such as oxygen, carbon, nitrogen, and sulfur. There are two ice giants in the Solar System: Uranus and Neptune. In astrophysics and planetary science the term "ice" refers to volatile chemical compounds with freezing points above about 100 K, such as water, ammonia, or methane, with freezing points of 273 K (0 °C), 195 K (−78 °C), and 91 K (−182 °C), respectively. In the 1990s, it was determined that Uranus and Neptune were a distinct class of giant planet, separate from the other giant planets, Jupiter and Saturn, which are gas giants predominantly composed of hydrogen and helium. Neptune and Uranus are now referred to as ''ice giants''. Lacking well-defined solid surfaces, they are primarily composed of gases and liquids. Their constituent compounds were solids when they were primarily incorporated into the planets during their formation, ei ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ortho Acid
In organic chemistry, ortho acids are organic, hypothetical chemical compounds having the structure (R = alkyl or aryl). Ortho acids themselves are unstable and cannot be isolated. However, ortho esters can be synthesized by the Pinner reaction, in which nitriles react with alcohols under acid catalysis: : Historic definition Historically the prefixes "''hypo''-", "''per''-", "''ortho''-", "''meta-''", and "''pyro''-" were used to distinguish between different oxyacids of the same element, of these ortho acid is the most highly oxidised or hydroxylated. For example, dehydration of orthoperiodic acid gives metaperiodic acid. Such naming conventions are now obsolete; however, various traditional names containing these prefixes have been retained in IUPAC nomenclature, namely: *orthosilicic acid, * orthotelluric acid, *orthophosphoric acid, * orthoboric acid, Hypothetical chemical compounds *orthoacetic acid, *orthocarbonic acid, *orthoformic acid Orthoformic acid or meth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
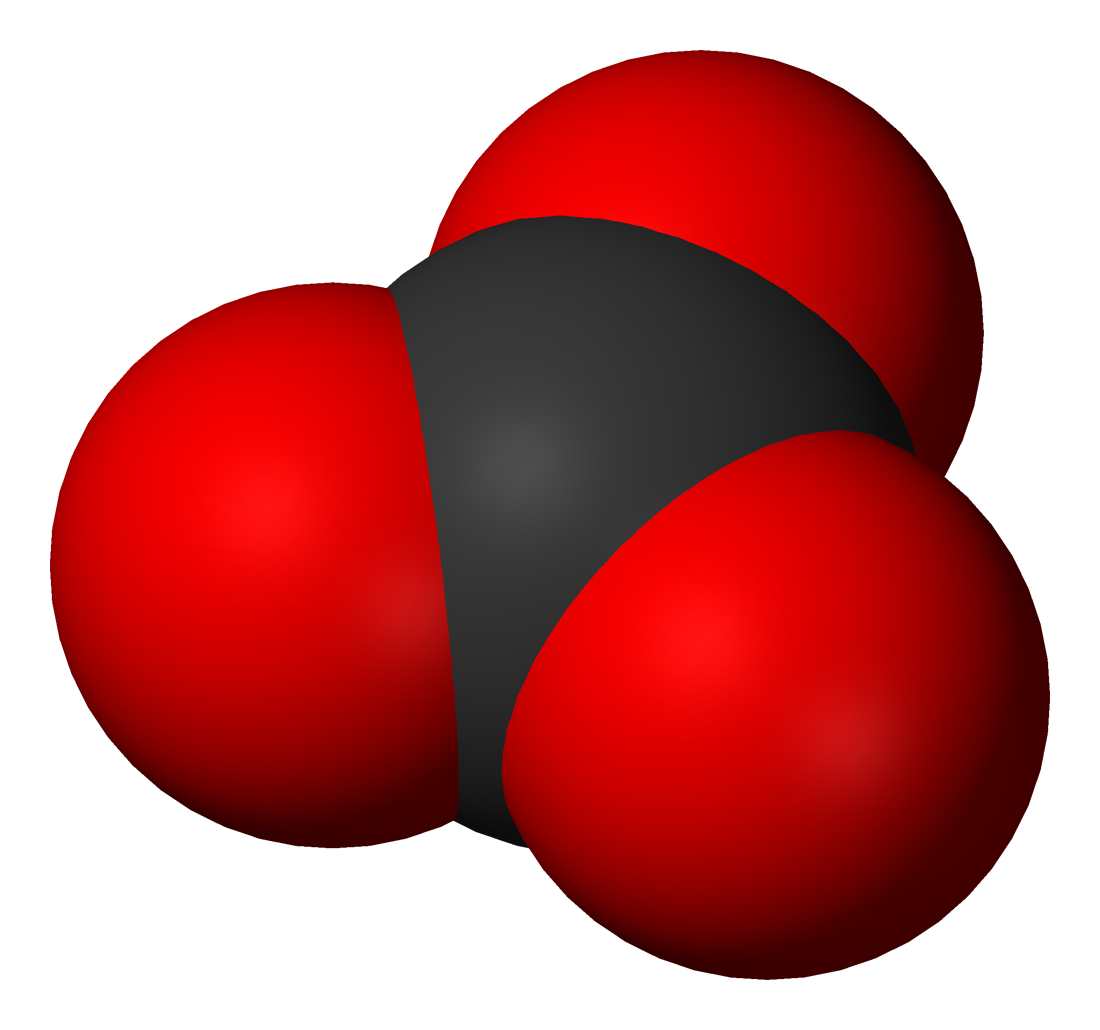
Carbonic Acid
Carbonic acid is a chemical compound with the chemical formula . The molecule rapidly converts to water and carbon dioxide in the presence of water. However, in the absence of water, it is quite stable at room temperature. The interconversion of carbon dioxide and carbonic acid is related to the breathing cycle of animals and the acidification of natural waters. In biochemistry and physiology, the name "carbonic acid" is sometimes applied to aqueous solutions of carbon dioxide. These chemical species play an important role in the bicarbonate buffer system, used to maintain acid–base homeostasis. Terminology in biochemical literature In chemistry, the term "carbonic acid" strictly refers to the chemical compound with the formula . Some biochemistry literature effaces the distinction between carbonic acid and carbon dioxide dissolved in extracellular fluid. In physiology, carbon dioxide excreted by the lungs may be called ''volatile acid'' or ''respiratory acid''. Anh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxoacid
An oxyacid, oxoacid, or ternary acid is an acid that contains oxygen. Specifically, it is a compound that contains hydrogen, oxygen, and at least one other element, with at least one hydrogen atom bonded to oxygen that can dissociate to produce the H+ cation and the anion of the acid. Description Under Lavoisier's original theory, all acids contained oxygen, which was named from . It was later discovered that some acids, notably hydrochloric acid, did not contain oxygen and so acids were divided into oxo-acids and these new hydroacids. All oxyacids have the acidic hydrogen bound to an oxygen atom, so bond strength (length) is not a factor, as it is with binary nonmetal hydrides. Rather, the electronegativity of the central atom and the number of oxygen atoms determine oxyacid acidity. For oxyacids with the same central atom, acid strength increases with the number of oxygen atoms attached to it. With the same number of oxygen atoms attached to it, acid strength increases with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxocarbon Anion
In chemistry, an oxocarbon anion is a negative ion consisting solely of carbon and oxygen atoms, and therefore having the general formula for some integers ''x'', ''y'', and ''n''. The most common oxocarbon anions are carbonate, , and oxalate, . There are however a large number of stable anions in this class, including several ones that have research or industrial use. There are also many unstable anions, like and , that have a fleeting existence during some chemical reactions; and many hypothetical species, like , that have been the subject of theoretical studies but have yet to be observed. Stable oxocarbon anions form salts with a large variety of cations. Unstable anions may persist in very rarefied gaseous state, such as in interstellar clouds. Most oxocarbon anions have corresponding moieties in organic chemistry, whose compounds are usually esters. Thus, for example, the oxalate moiety occurs in the ester dimethyl oxalate . Electronic structure of the carbonat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protons
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' ( elementary charge). Its mass is slightly less than the mass of a neutron and approximately times the mass of an electron (the proton-to-electron mass ratio). Protons and neutrons, each with a mass of approximately one dalton, are jointly referred to as '' nucleons'' (particles present in atomic nuclei). One or more protons are present in the nucleus of every atom. They provide the attractive electrostatic central force which binds the atomic electrons. The number of protons in the nucleus is the defining property of an element, and is referred to as the atomic number (represented by the symbol ''Z''). Since each element is identified by the number of protons in its nucleus, each element has its own atomic number, which determines the number of atomic electrons and consequently the chemical characteristics of the element. The word ''proton'' is Greek for "first ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alcohol (chemistry)
In chemistry, an alcohol (), is a type of organic compound that carries at least one hydroxyl () functional group bound to a Saturated and unsaturated compounds, saturated carbon atom. Alcohols range from the simple, like methanol and ethanol, to complex, like sugar alcohols and cholesterol. The presence of an OH group strongly modifies the properties of Hydrocarbon, hydrocarbons, conferring Hydrophile, hydrophilic (water-loving) properties. The OH group provides a site at which many reactions can occur. History The flammable nature of the exhalations of wine was already known to ancient natural philosophers such as Aristotle (384–322 BCE), Theophrastus (–287 BCE), and Pliny the Elder (23/24–79 CE). However, this did not immediately lead to the isolation of alcohol, even despite the development of more advanced distillation techniques in second- and third-century Roman Egypt. An important recognition, first found in one of the writings attributed to Jabir ibn Hayyan, J� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy groups. Both the negatively charged anion , called hydroxide, and the neutral radical , known as the hydroxyl radical, consist of an unbonded hydroxy group. According to IUPAC definitions, the term ''hydroxyl'' refers to the hydroxyl radical () only, while the functional group is called a ''hydroxy group''. Properties Water, alcohols, carboxylic acids, and many other hydroxy-containing compounds can be readily deprotonated due to a large difference between the electronegativity of oxygen (3.5) and that of hydrogen (2.1). Hydroxy-containing compounds engage in intermolecular hydrogen bonding increasing the electrostatic attraction between molecules and thus to higher boiling and melting points than found for compounds that lack thi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Atom
Carbon () is a chemical element; it has symbol C and atomic number 6. It is nonmetallic and tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 electrons. It belongs to group 14 of the periodic table. Carbon makes up about 0.025 percent of Earth's crust. Three isotopes occur naturally, C and C being stable, while C is a radionuclide, decaying with a half-life of 5,700 years. Carbon is one of the few elements known since antiquity. Carbon is the 15th most abundant element in the Earth's crust, and the fourth most abundant element in the universe by mass after hydrogen, helium, and oxygen. Carbon's abundance, its unique diversity of organic compounds, and its unusual ability to form polymers at the temperatures commonly encountered on Earth, enables this element to serve as a common element of all known life. It is the second most abundant element in the human body by mass (about 18.5%) after oxygen. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Formula
A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and ''plus'' (+) and ''minus'' (−) signs. These are limited to a single typographic line of symbols, which may include subscripts and superscripts. A chemical formula is not a chemical name since it does not contain any words. Although a chemical formula may imply certain simple chemical structures, it is not the same as a full chemical structural formula. Chemical formulae can fully specify the structure of only the simplest of molecules and chemical substances, and are generally more limited in power than chemical names and structural formulae. The simplest types of chemical formulae are called '' empirical formulae'', which use letters and numbers indicating the numerical ''proportions'' of atoms ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |