triphenylphosphine on:
[Wikipedia]
[Google]
[Amazon]
Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to P Ph3 or Ph3P. It is versatile compound that is widely used as a reagent in
International Chemical Safety Card 0700
Tertiary phosphines Phenyl compounds
organic synthesis
Organic synthesis is a branch of chemical synthesis concerned with the construction of organic compounds. Organic compounds are molecules consisting of combinations of covalently-linked hydrogen, carbon, oxygen, and nitrogen atoms. Within the gen ...
and as a ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
for transition metal complexes, including ones that serve as catalysts in organometallic chemistry
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and so ...
. PPh3 exists as relatively air stable, colorless crystals at room temperature. It dissolves in non-polar organic solvents such as benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hyd ...
and diethyl ether
Diethyl ether, or simply ether, is an organic compound with the chemical formula , sometimes abbreviated as . It is a colourless, highly Volatility (chemistry), volatile, sweet-smelling ("ethereal odour"), extremely flammable liquid. It belongs ...
.
Preparation and structure
Triphenylphosphine can be prepared in the laboratory by treatment ofphosphorus trichloride
Phosphorus trichloride is an inorganic compound with the chemical formula PCl3. A colorless liquid when pure, it is an important industrial chemical, being used for the manufacture of phosphites and other organophosphorus compounds. It is toxic ...
with phenylmagnesium bromide
Phenylmagnesium bromide, with the simplified formula , is a magnesium-containing organometallic compound. It forms colorless crystals. It is commercially available as a solution in diethyl ether or tetrahydrofuran (THF). Phenylmagnesium bromide i ...
or phenyllithium
Phenyllithium is an organometallic agent with the empirical formula . It is most commonly used as a metalating agent in organic syntheses and a substitute for Grignard reagents for introducing phenyl groups in organic syntheses. Crystalline phenyl ...
. The industrial synthesis involves the reaction between phosphorus trichloride
Phosphorus trichloride is an inorganic compound with the chemical formula PCl3. A colorless liquid when pure, it is an important industrial chemical, being used for the manufacture of phosphites and other organophosphorus compounds. It is toxic ...
, chlorobenzene
Chlorobenzene (abbreviated PhCl) is an aryl chloride and the simplest of the chlorobenzenes, consisting of a benzene ring substituted with one chlorine atom. Its chemical formula is C6H5Cl. This colorless, flammable liquid is a common solvent a ...
, and sodium:
:PCl3 + 3 PhCl + 6 Na → PPh3 + 6 NaCl
Triphenylphosphine crystallizes in triclinic and monoclinic modification. In both cases, the molecule adopts a pyramidal structure with propeller-like arrangement of the three phenyl groups.
Principal reactions with chalcogens, halogens, and acids
Oxidation
Triphenylphosphine undergoes slow oxidation by air to give triphenylphosphine oxide, Ph3PO: :2 PPh3 + O2 → 2 OPPh3 This impurity can be removed by recrystallisation of PPh3 from either hotethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the ps ...
or isopropanol
Isopropyl alcohol (IUPAC name propan-2-ol and also called isopropanol or 2-propanol) is a colorless, flammable, organic compound with a pungent alcoholic odor.
Isopropyl alcohol, an organic polar molecule, is miscible in water, ethanol, an ...
. This method capitalizes on the fact that OPPh3 is more polar and hence more soluble in polar solvents than PPh3.
Triphenylphosphine abstracts sulfur
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms ...
from polysulfide
Polysulfides are a class of chemical compounds derived from anionic chains of sulfur atoms. There are two main classes of polysulfides: inorganic and organic. The inorganic polysulfides have the general formula . These anions are the conjugate bas ...
compounds, episulfides, and elemental sulfur
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms ...
. Simple organosulfur compounds such as thiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl grou ...
s and thioether
In organic chemistry, a sulfide (British English sulphide) or thioether is an organosulfur functional group with the connectivity as shown on right. Like many other sulfur-containing compounds, Volatile organic compound, volatile sulfides have ...
s are unreactive, however. The phosphorus-containing product is triphenylphosphine sulfide, Ph3PS. This reaction can be employed to assay the "labile" S0 content of a sample, say vulcanized rubber. Triphenylphosphine selenide, Ph3PSe, may be easily prepared via treatment of PPh3 with red (alpha-monoclinic) Se. Salts of selenocyanate, SeCN−, are used as the Se0 source. PPh3 can also form an adduct with Te, although this adduct primarily exists as (Ph3P)2Te rather than PPh3Te.
Aryl azides
In chemistry, azide (, ) is a Linear molecular geometry, linear, polyatomic anion with the Chemical formula, formula and Chemical structure, structure . It is the conjugate base of hydrazoic acid . Organic azides are organic compounds with the ...
react with PPh3 to give phosphanimines, analogues of OPPh3, via the Staudinger reaction. Illustrative is the preparation of triphenylphosphine phenylimide:
:PPh3 + PhN3 → PhNPPh3 + N2
The phosphanimine can be hydrolyzed to the amine. Typically the intermediate phosphanimine is not isolated.
:PPh3 + RN3 + H2O → OPPh3 + N2 + RNH2
Chlorination
Cl2 adds to PPh3 to give triphenylphosphine dichloride ( Ph3Cll), which exists as the moisture-sensitive phosphonium halide. This reagent is used to convertalcohol
Alcohol may refer to:
Common uses
* Alcohol (chemistry), a class of compounds
* Ethanol, one of several alcohols, commonly known as alcohol in everyday life
** Alcohol (drug), intoxicant found in alcoholic beverages
** Alcoholic beverage, an alco ...
s to alkyl chlorides in organic synthesis
Organic synthesis is a branch of chemical synthesis concerned with the construction of organic compounds. Organic compounds are molecules consisting of combinations of covalently-linked hydrogen, carbon, oxygen, and nitrogen atoms. Within the gen ...
. Bis(triphenylphosphine)iminium chloride (PPN+Cl−, formula C6H5)3P)2Nl is prepared from triphenylphosphine dichloride:
:2 Ph3PCl2 + NH2OH·HCl + Ph3P → Cl + 4HCl + Ph3PO
Protonation
PPh3 is a weak base (aqueous p''K''aH = 2.73, determined electrochemically), although it is a considerably stronger base than NPh3 (estimated aqueous p''K''aH < −3). It forms isolable triphenylphosphonium salts with strong acids such as HBr:Hercouet, A.; LeCorre, M. (1988) Triphenylphosphonium bromide: A convenient and quantitative source of gaseous hydrogen bromide. Synthesis, 157–158 :P(C6H5)3 + HBr → P(C6H5)3sup>+Br−Organic reactions
PPh3 is widely used inorganic synthesis
Organic synthesis is a branch of chemical synthesis concerned with the construction of organic compounds. Organic compounds are molecules consisting of combinations of covalently-linked hydrogen, carbon, oxygen, and nitrogen atoms. Within the gen ...
. The properties that guide its usage are its nucleophilicity and its reducing character. The nucleophilicity of PPh3 is indicated by its reactivity toward electrophilic alkenes, such as Michael-acceptors, and alkyl halides. It is also used in the synthesis of biaryl compounds, such as the Suzuki reaction
The Suzuki reaction or Suzuki coupling is an organic reaction that uses a palladium complex catalyst to cross-couple a boronic acid to an organohalide. It was first published in 1979 by Akira Suzuki, and he shared the 2010 Nobel Prize in Chemi ...
.
Quaternization
PPh3 combines withalkyl halide
The haloalkanes (also known as halogenoalkanes or alkyl halides) are alkanes containing one or more halogen substituents of hydrogen atom. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalka ...
s to give phosphonium salts. This quaternization reaction is particularly fast for benzylic and allylic halides:
:PPh3 + CH3I → H3PPh3sup>+I−
These salts, which can often be isolated as crystalline solids, react with strong bases to form ylide
An ylide () or ylid () is a neutral dipolar molecule containing a formally negatively charged atom (usually a carbanion) directly attached to a heteroatom with a formal positive charge (usually nitrogen, phosphorus or sulfur), and in which both ...
s, which are reagents in the Wittig reaction
The Wittig reaction or Wittig olefination is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide called a Wittig reagent. Wittig reactions are most commonly used to convert aldehydes and ketones to alkenes. Most o ...
s.
Aryl halides will quaternize PPh3 to give tetraphenylphosphonium salts:
:PPh3 + PhBr → Ph4r
The reaction however requires elevated temperatures and metal catalysts.
Mitsunobu reaction
In theMitsunobu reaction
The Mitsunobu reaction is an organic reaction that converts an alcohol into a variety of functional groups, such as an ester, using triphenylphosphine and an azodicarboxylate such as diethyl azodicarboxylate (DEAD) or diisopropyl azodicarboxy ...
, a mixture of triphenylphosphine and diisopropyl azodicarboxylate ("DIAD", or its diethyl analogue, DEAD
Death is the end of life; the irreversible cessation of all biological functions that sustain a living organism. Death eventually and inevitably occurs in all organisms. The remains of a former organism normally begin to decompose sho ...
) converts an alcohol and a carboxylic acid to an ester. DIAD is reduced as it serves as the hydrogen acceptor, and the PPh3 is oxidized to OPPh3.
Appel reaction
In the Appel reaction, a mixture of PPh3 and CX4 (X = Cl, Br) is used to convert alcohols to alkyl halides. Triphenylphosphine oxide (OPPh3) is a byproduct. :PPh3 + CBr4 + RCH2OH → OPPh3 + RCH2Br + HCBr3 This reaction commences with nucleophilic attack of PPh3 on CBr4, an extension of the quaternization reaction listed above.Deoxygenation
The easy oxygenation of PPh3 is exploited in its use to deoxygenate organic peroxides, which generally occurs with retention of configuration: :PPh3 + RO2H → OPPh3 + ROH (R = alkyl) It is also used for the decomposition of organicozonide
Ozonide is the polyatomic anion . Cyclic organic compounds formed by the addition of ozone () to an alkene are also called ozonides.
Ionic ozonides
Inorganic ozonides are dark red salts. The anion has the bent shape of the ozone molecule.
In ...
s to ketones and aldehydes, although dimethyl sulfide
Dimethyl sulfide (DMS) or methylthiomethane is an organosulfur compound with the formula . It is the simplest thioether and has a characteristic disagreeable odor. It is a flammable liquid that boils at . It is a component of the smell produc ...
is more popular for the reaction as the side product, dimethyl sulfoxide
Dimethyl sulfoxide (DMSO) is an organosulfur compound with the formula . This colorless liquid is the sulfoxide most widely used commercially. It is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is ...
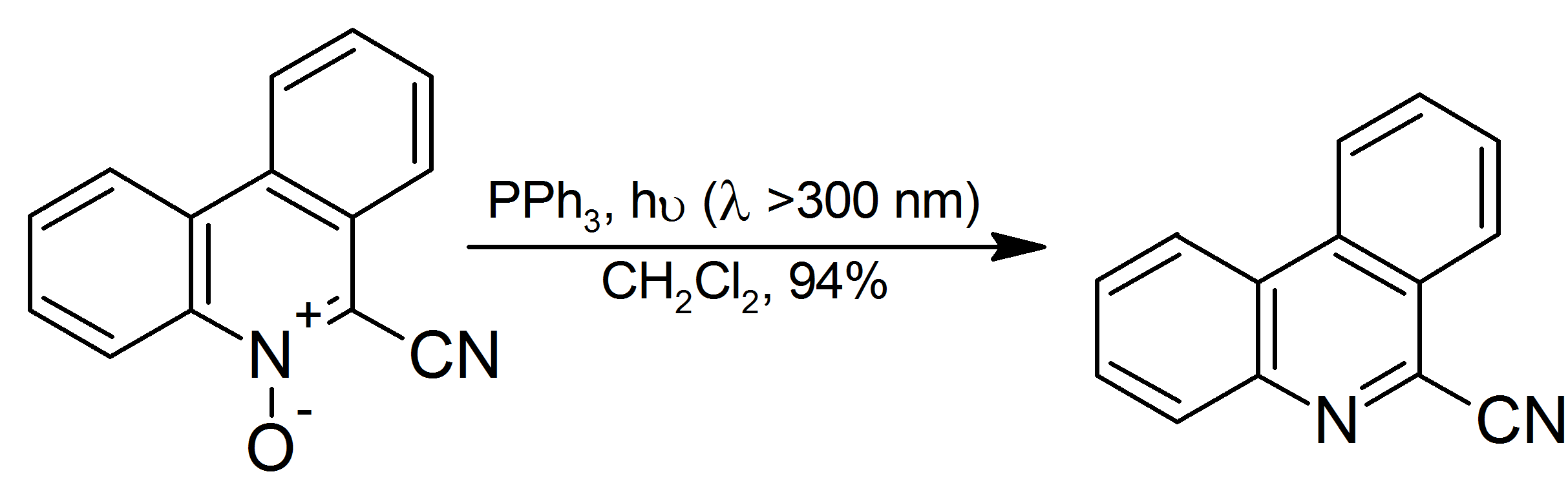
is more readily separated from the reaction mixture than triphenylphosphine oxide. Aromatic ''N''-oxides are reduced to the corresponding amine in high yield at room temperature with irradiation:
:
Sulfonation
Sulfonation
In organic chemistry, aromatic sulfonation is a reaction in which a hydrogen atom on an arene is replaced by a sulfonic acid () group. Together with nitration and chlorination, aromatic sulfonation is a widely used electrophilic aromatic substi ...
of PPh3 gives tris(3-sulfophenyl)phosphine, P(C6H4-3-SO3−)3 ( TPPTS), usually isolated as the trisodium salt. In contrast to PPh3, TPPTS is water-soluble, as are its metal derivatives. Rhodium complexes of TPPTS are used in certain industrial hydroformylation
In organic chemistry, hydroformylation, also known as oxo synthesis or oxo process, is an industrial process for the production of aldehydes () from alkenes (). This chemical reaction entails the net addition of a formyl group () and a hydrogen ...
reactions.
Reduction to diphenylphosphide
Lithium
Lithium (from , , ) is a chemical element; it has chemical symbol, symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard temperature and pressure, standard conditions, it is the least dense metal and the ...
in THF as well as Na or K react with PPh3 to give Ph2PM (M = Li, Na, K). These salts are versatile precursors to tertiary phosphines. For example, 1,2-dibromoethane
1,2-Dibromoethane, also known as ethylene dibromide (EDB), is an organobromine compound with the chemical formula . Although trace amounts occur naturally in the ocean, where it is probably formed by algae and kelp, substantial amounts are produc ...
and Ph2PM react to give Ph2PCH2CH2PPh2. Weak acids such ammonium chloride
Ammonium chloride is an inorganic chemical compound with the chemical formula , also written as . It is an ammonium salt of hydrogen chloride. It consists of ammonium cations and chloride anions . It is a white crystalline salt (chemistry), sal ...
, convert Ph2PM (M = Li, Na, K) into diphenylphosphine:
:(C6H5)2PM + H2O → (C6H5)2PH + MOH
Transition metal complexes
Triphenylphosphine binds well to mosttransition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. The lanthanide and actinid ...
s, especially those in the middle and late transition metals of groups 7–10. In terms of steric bulk, PPh3 has a Tolman cone angle of 145°, which is intermediate between those of P(C6H11)3 (170°) and P(CH3)3 (115°). In an early application in homogeneous catalysis
In chemistry, homogeneous catalysis is catalysis where the catalyst is in same phase as reactants, principally by a soluble catalyst in a solution. In contrast, heterogeneous catalysis describes processes where the catalysts and substrate are in d ...
, NiBr2(PPh3)2 was used by Walter Reppe for the synthesis of acrylate esters from alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
s, carbon monoxide
Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
, and alcohol
Alcohol may refer to:
Common uses
* Alcohol (chemistry), a class of compounds
* Ethanol, one of several alcohols, commonly known as alcohol in everyday life
** Alcohol (drug), intoxicant found in alcoholic beverages
** Alcoholic beverage, an alco ...
s.*{{cite journal , title = Cyclisierende Polymerisation von Acetylen. III Benzol, Benzolderivate und hydroaromatische Verbindungen , author1=Reppe, W. , author2=Schweckendiek, W. J. , journal = Justus Liebigs Annalen der Chemie , volume = 560 , issue = 1 , pages = 104–116 , year = 1948 , doi = 10.1002/jlac.19485600104 The use of PPh3 was popularized by its use in the hydroformylation
In organic chemistry, hydroformylation, also known as oxo synthesis or oxo process, is an industrial process for the production of aldehydes () from alkenes (). This chemical reaction entails the net addition of a formyl group () and a hydrogen ...
catalyst RhH(PPh3)3(CO).
Polymer-anchored PPh3 derivatives
Polymeric analogues of PPh3 are known whereby polystyrene is modified with PPh2 groups at the para position. Such polymers can be employed in many of the applications used for PPh3 with the advantage that the polymer, being insoluble, can be separated from products by simple filtration of reaction slurries. Such polymers are prepared via treatment of 4-lithiophenyl-substituted polystyrene withchlorodiphenylphosphine
Chlorodiphenylphosphine is an organophosphorus compound with the formula (C6H5)2PCl, abbreviated Ph2PCl. It is a colourless oily liquid with a pungent odor that is often described as being garlic-like and detectable even in the ppb range. It is ...
(PPh2Cl).
See also
* Tris(o-tolyl)phosphine * Decyl(triphenyl)phosphonium * Vaska's complex * Wilkinson's catalyst * Bis(triphenylphosphine)nickel(II) dichloride *Bis(triphenylphosphine)palladium(II) dichloride
Bis(triphenylphosphine)palladium chloride is a coordination compound of palladium containing two triphenylphosphine and two chloride ligands. It is a yellow solid that is soluble in some organic solvents. It is used for palladium-catalyzed coupli ...
* Stryker's reagent
References
External links
International Chemical Safety Card 0700
Tertiary phosphines Phenyl compounds