|
Tetrafluorohydrazine
Tetrafluorohydrazine or perfluorohydrazine, , is a colourless, nonflammable, reactive inorganic gas. It is a fluorinated analog of hydrazine. Synthesis Tetrafluorohydrazine was originally prepared from nitrogen trifluoride using a copper as a fluorine atom acceptor: : A number of F-atom acceptors can be used, including carbon, other metals, and nitric oxide. These reactions exploit the relatively weak N-F bond in NF3. Properties Tetrafluorohydrazine is in equilibrium with its radical monomer nitrogen difluoride. : At room temperature is mostly associated with only 0.7% in the form of at 5mm Hg pressure. When the temperature rises to 225 °C, it mostly dissociates with 99% in the form of . molecule dimensions and angles The energy needed to break the N−N bond in is 20.8 kcal/mol, with an entropy change of 38.6 eu. For comparison, the dissociation energy of the N−N bond is in , in , and in . The enthalpy of formation of (Δf''H''°) is 34.421 kJ/mo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrazine
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly hazardous unless handled in solution as, for example, hydrazine hydrate (). Hydrazine is mainly used as a foaming agent in preparing Polymeric foam, polymer foams, but applications also include its uses as a precursor (chemistry), precursor to pharmaceuticals and agrochemicals, as well as a long-term storable propellant for in-outer space, space spacecraft propulsion. Additionally, hydrazine is used in various rocket propellant, rocket fuels and to prepare the gas precursors used in airbags. Hydrazine is used within both nuclear and conventional electrical power plant steam cycles as an oxygen scavenger to control concentrations of dissolved oxygen in an effort to reduce corrosion. , approximately 120,000 tons of hydrazine hydrate (corresponding to a 64% solution of hydrazine in water by weight) we ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
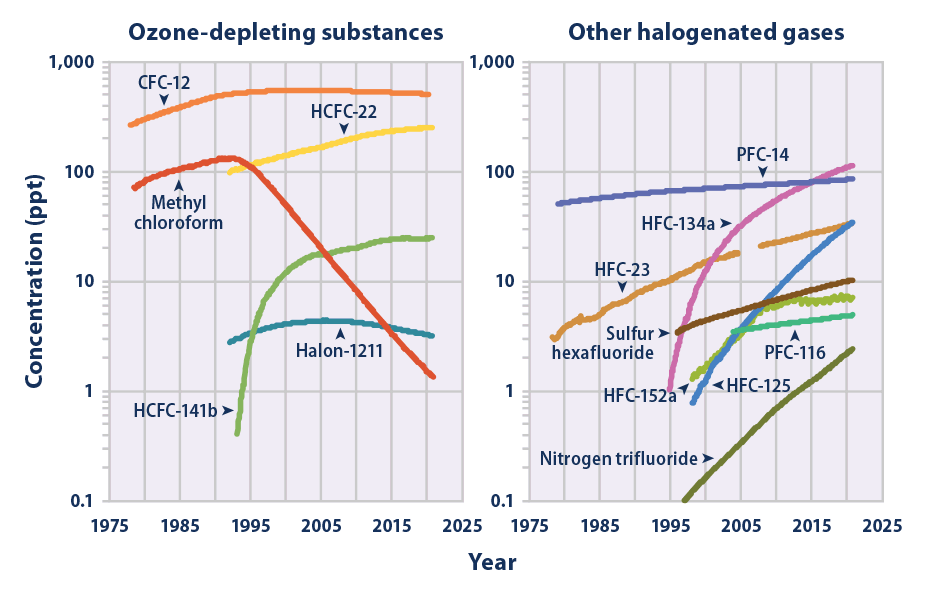
Nitrogen Trifluoride
Nitrogen trifluoride is the inorganic compound with the formula (). It is a colorless, non-flammable, toxic gas with a slightly musty odor. In contrast with ammonia, it is nonbasic. It finds increasing use within the manufacturing of flat-panel displays, photovoltaics, LEDs and other microelectronics. is a greenhouse gas, with a global warming potential (GWP) 17,200 times greater than that of when compared over a 100-year period. Synthesis and reactivity Nitrogen trifluoride can be prepared from the elements in the presence of an electric discharge. In 1903, Otto Ruff prepared nitrogen trifluoride by the electrolysis of a molten mixture of ammonium fluoride and hydrogen fluoride. It is far less reactive than the other nitrogen trihalides nitrogen trichloride, nitrogen tribromide, and nitrogen triiodide, all of which are explosive. Alone among the nitrogen trihalides it has a negative enthalpy of formation. It is prepared in modern times both by direct reaction of a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen Difluoride
Nitrogen difluoride, also known as difluoroamino, is a reactive radical molecule with formula . This small molecule is in equilibrium with its dimer tetrafluorohydrazine. : As the temperature increases the proportion of increases. The molecule is unusual in that it has an odd number of electrons, yet is stable enough to study experimentally. Properties The energy needed to break the N–N bond in is , with an entropy change of 38.6 eu. molecule dimensions and angles For comparison, the dissociation energy of the N–N bond is in , in , and in . The enthalpy of formation of (Δf''H'') is . At room temperature is mostly associated with only 0.7% in the form of at pressure. When the temperature rises to 225 °C, it mostly dissociates with 99% in the form of . In , the N–F bond length is 1.3494 Å and the angle subtended at F–N–F is 103.33°. In the infrared spectrum the N–F bond in has a symmetrical stretching frequency of 1075 cm−1. T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Compound
An inorganic compound is typically a chemical compound that lacks carbon–hydrogen bondsthat is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as ''inorganic chemistry''. Inorganic compounds comprise most of the Earth's crust, although the compositions of the deep Mantle (geology), mantle remain active areas of investigation. All allotropes (structurally different pure forms of an element) and some simple carbon compounds are often considered inorganic. Examples include the allotropes of carbon (graphite, diamond, buckminsterfullerene, graphene, etc.), carbon monoxide , carbon dioxide , carbides, and salt (chemistry), salts of inorganic anions such as carbonates, cyanides, cyanates, thiocyanates, isothiocyanates, etc. Many of these are normal parts of mostly organic systems, including organisms; describing a chemical as inorganic does not necessarily mean that it cannot occur within life, living things. History ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neurotoxin
Neurotoxins are toxins that are destructive to nervous tissue, nerve tissue (causing neurotoxicity). Neurotoxins are an extensive class of exogenous chemical neurological insult (medical), insultsSpencer 2000 that can adversely affect function in both developing and mature nervous tissue.Olney 2002 The term can also be used to classify endogenous compounds, which, when abnormally contacted, can prove neurologically toxic. Though neurotoxins are often neurologically destructive, their ability to specifically target neural components is important in the study of nervous systems. Common examples of neurotoxins include lead, ethanol (drinking alcohol), glutamate,Choi 1987 nitric oxide, botulinum toxin (e.g. Botox), tetanus toxin,Simpson 1986 and tetrodotoxin. Some substances such as nitric oxide and glutamate are in fact essential for proper function of the body and only exert neurotoxic effects at excessive concentrations. Neurotoxins inhibit neuron control over ion concentrations ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methemoglobinemia
Methemoglobinemia, or methaemoglobinaemia, is a condition of elevated methemoglobin in the blood. Symptoms may include headache, dizziness, shortness of breath, nausea, poor muscle coordination, and blue-colored skin (cyanosis). Complications may include seizures and heart arrhythmias. Methemoglobinemia can be due to certain medications, chemicals, or food or it can be inherited. Substances involved may include benzocaine, nitrites, or dapsone. The underlying mechanism involves some of the iron in hemoglobin being converted from the ferrous [Fe2+] to the ferric [Fe3+] form. The diagnosis is often suspected based on symptoms and a Hypoxia (medical), low blood oxygen that does not improve with oxygen therapy. Diagnosis is confirmed by a blood gas. Treatment is generally with oxygen therapy and methylene blue. Other treatments may include vitamin C, exchange transfusion, and hyperbaric oxygen therapy. Outcomes are generally good with treatment. Methemoglobinemia is relatively unc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidizing Agent
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or " accepts"/"receives" an electron from a (called the , , or ''electron donor''). In other words, an oxidizer is any substance that oxidizes another substance. The oxidation state, which describes the degree of loss of electrons, of the oxidizer decreases while that of the reductant increases; this is expressed by saying that oxidizers "undergo reduction" and "are reduced" while reducers "undergo oxidation" and "are oxidized". Common oxidizing agents are oxygen, hydrogen peroxide, and the halogens. In one sense, an oxidizing agent is a chemical species that undergoes a chemical reaction in which it gains one or more electrons. In that sense, it is one component in an oxidation–reduction (redox) reaction. In the second sense, an oxidizing agent is a chemical species that transfers electronegative atoms, usually oxygen, t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liquefied Gas
Liquefied gas (sometimes referred to as liquid gas) is a gas that has been turned into a liquid by cooling or compressing it. Examples of liquefied gases include liquid air, liquefied natural gas, and liquefied petroleum gas. Liquid air At the Lister Institute of Preventive Medicine, liquid air has been brought into use as an agent in biological research. An inquiry into the intracellular constituents of the typhoid bacillus, initiated under the direction of Doctor Allan Macfadyen, necessitated the separation of the cell-plasma of the organism. The method at first adopted for the disintegration of the bacteria was to mix them with silver-sand and churn the whole up in a closed vessel in which a series of horizontal vanes revolved at a high speed. But certain disadvantages attached to this procedure, and accordingly some means was sought to do away with the sand and triturate the bacilli per se. This was found in liquid air, which, as had long before been shown at the Royal Insti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reducing Agents
In chemistry, a reducing agent (also known as a reductant, reducer, or electron donor) is a chemical species that "donates" an electron to an (called the , , , or ). Examples of substances that are common reducing agents include hydrogen, carbon monoxide, the alkali metals, formic acid, oxalic acid, and sulfite compounds. In their pre-reaction states, reducers have extra electrons (that is, they are by themselves reduced) and oxidizers lack electrons (that is, they are by themselves oxidized). This is commonly expressed in terms of their oxidation states. An agent's oxidation state describes its degree of loss of electrons, where the higher the oxidation state then the fewer electrons it has. So initially, prior to the reaction, a reducing agent is typically in one of its lower possible oxidation states; its oxidation state increases during the reaction while that of the oxidizer decreases. Thus in a redox reaction, the agent whose oxidation state increases, that "loses/Electron d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter. Under standard conditions, hydrogen is a gas of diatomic molecules with the chemical formula, formula , called dihydrogen, or sometimes hydrogen gas, molecular hydrogen, or simply hydrogen. Dihydrogen is colorless, odorless, non-toxic, and highly combustible. Stars, including the Sun, mainly consist of hydrogen in a plasma state, while on Earth, hydrogen is found as the gas (dihydrogen) and in molecular forms, such as in water and organic compounds. The most common isotope of hydrogen (H) consists of one proton, one electron, and no neutrons. Hydrogen gas was first produced artificially in the 17th century by the reaction of acids with metals. Henry Cavendish, in 1766–1781, identified hydrogen gas as a distinct substance and discovere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Room Temperature
Room temperature, colloquially, denotes the range of air temperatures most people find comfortable indoors while dressed in typical clothing. Comfortable temperatures can be extended beyond this range depending on humidity, air circulation, and other factors. In certain fields, like science and engineering, and within a particular context, room temperature can mean different agreed-upon ranges. In contrast, ambient temperature is the actual temperature, as measured by a thermometer, of the air (or other medium and surroundings) in any particular place. The ambient temperature (e.g. an unheated room in winter) may be very different from an ideal ''room temperature''. Food and beverages may be served at "room temperature", meaning neither heated nor cooled. Comfort temperatures Comfort temperature is interchangeable with neutral temperature in the scientific literature, which can be calculated through regression analysis between thermal sensation votes and indoor temperature. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |