Nitrogen Trifluoride on:
[Wikipedia]
[Google]
[Amazon]
Nitrogen trifluoride is the
 The GWP of is second only to in the group of Kyoto-recognised greenhouse gases, and was included in that grouping with effect from 2013 and the commencement of the second commitment period of the Kyoto Protocol. It has an estimated atmospheric lifetime of 740 years, although other work suggests a slightly shorter lifetime of 550 years (and a corresponding GWP of 16,800).
The GWP of is second only to in the group of Kyoto-recognised greenhouse gases, and was included in that grouping with effect from 2013 and the commencement of the second commitment period of the Kyoto Protocol. It has an estimated atmospheric lifetime of 740 years, although other work suggests a slightly shorter lifetime of 550 years (and a corresponding GWP of 16,800).
 Since 1992, when less than 100 tons were produced, production grew to an estimated 4000 tons in 2007 and is projected to increase significantly. World production of NF3 is expected to reach 8000 tons a year by 2010. By far the world's largest producer of is the US
Since 1992, when less than 100 tons were produced, production grew to an estimated 4000 tons in 2007 and is projected to increase significantly. World production of NF3 is expected to reach 8000 tons a year by 2010. By far the world's largest producer of is the US
NF3
Code of Practice (European Industrial Gas Association)]
WebBook page for NF3
{{DEFAULTSORT:Nitrogen Trifluoride Inorganic amines Nitrogen fluorides Greenhouse gases Industrial gases Nitrogen(III) compounds
inorganic compound
An inorganic compound is typically a chemical compound that lacks carbon–hydrogen bondsthat is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as ''inorganic chemistry''.
Inorgan ...
with the formula (). It is a colorless, non-flammable
A combustible material is a material that can burn (i.e., sustain a flame) in air under certain conditions. A material is flammable if it ignites easily at ambient temperatures. In other words, a combustible material ignites with some effort ...
, toxic
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a subst ...
gas with a slightly musty odor. In contrast with ammonia, it is nonbasic. It finds increasing use within the manufacturing of flat-panel displays, photovoltaics, LEDs and other microelectronics
Microelectronics is a subfield of electronics. As the name suggests, microelectronics relates to the study and manufacture (or microfabrication) of very small electronic designs and components. Usually, but not always, this means micrometre ...
. is a greenhouse gas
Greenhouse gases (GHGs) are the gases in the atmosphere that raise the surface temperature of planets such as the Earth. Unlike other gases, greenhouse gases absorb the radiations that a planet emits, resulting in the greenhouse effect. T ...
, with a global warming potential
Global warming potential (GWP) is a measure of how much heat a greenhouse gas traps in the atmosphere over a specific time period, relative to carbon dioxide (). It is expressed as a multiple of warming caused by the same mass of carbon dioxide ( ...
(GWP) 17,200 times greater than that of when compared over a 100-year period.
Synthesis and reactivity
Nitrogen trifluoride can be prepared from the elements in the presence of an electric discharge. In 1903, Otto Ruff prepared nitrogen trifluoride by the electrolysis of a molten mixture of ammonium fluoride andhydrogen fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluori ...
. It is far less reactive than the other nitrogen trihalides nitrogen trichloride, nitrogen tribromide, and nitrogen triiodide, all of which are explosive. Alone among the nitrogen trihalides it has a negative enthalpy of formation. It is prepared in modern times both by direct reaction of ammonia and fluorine and by a variation of Ruff's method.Philip B. Henderson, Andrew J. Woytek "Fluorine Compounds, Inorganic, Nitrogen" in Kirk‑Othmer ''Encyclopedia of Chemical Technology'', 1994, John Wiley & Sons, NY. Article Online Posting Date: December 4, 2000 It is supplied in pressurized cylinders.
is slightly soluble in water without undergoing chemical reaction. It is nonbasic with a low dipole moment of 0.2340 D. By contrast, ammonia is basic and highly polar (1.47 D). This contrast reflects the differing electronegativities of H vs F.
Similar to dioxygen
There are several known allotropes of oxygen. The most familiar is molecular oxygen (), present at significant levels in Earth's atmosphere and also known as dioxygen or triplet oxygen. Another is the highly reactive ozone (). Others are:
* Ato ...
, NF3 is a potent yet sluggish oxidizer. It oxidizes hydrogen chloride to chlorine:
:2 NF3 + 6 HCl → 6 HF + N2 + 3 Cl2
However, it only attacks (explosively) organic compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-co ...
s at high temperatures. Consequently it is compatible under standard conditions with several plastics, as well as steel and Monel.
Above 200-300 °C, NF3 reacts with metals, carbon, and other reagents to give tetrafluorohydrazine:
:
NF3 reacts with fluorine and antimony pentafluoride
Antimony pentafluoride is the inorganic compound with the formula Sb F5. This colorless, viscous liquid is a strong Lewis acid and a component of the superacid fluoroantimonic acid, formed upon mixing liquid HF with liquid SbF5 in 1:1 ratio. It ...
to give the tetrafluoroammonium salt:
: NF3 + F2 + SbF5 → NFSbF
NF3 and B2H6 react vigorously even at cryogenic temperatures to give nitrogen gas
Nitrogen is a chemical element; it has symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh i ...
, boron trifluoride, and hydrofluoric acid
Hydrofluoric acid is a solution of hydrogen fluoride (HF) in water. Solutions of HF are colorless, acidic and highly corrosive. A common concentration is 49% (48–52%) but there are also stronger solutions (e.g. 70%) and pure HF has a boiling p ...
.
Applications
High-volume applications such asDRAM
Dram, DRAM, or drams may refer to:
Technology and engineering
* Dram (unit), a unit of mass and volume, and an informal name for a small amount of liquor, especially whisky or whiskey
* Dynamic random-access memory, a type of electronic semicondu ...
computer memory production, the manufacturing of flat panel displays and the large-scale production of thin-film solar cell
Thin-film solar cells are a type of solar cell made by depositing one or more thin layers (thin films or TFs) of photovoltaic material onto a substrate, such as glass, plastic or metal. Thin-film solar cells are typically a few nanometers (nan ...
s use .
Etching
Nitrogen trifluoride is primarily used to removesilicon
Silicon is a chemical element; it has symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid (sometimes considered a non-metal) and semiconductor. It is a membe ...
and silicon-compounds during the manufacturing of semiconductor devices such as LCD displays, some thin-film solar cell
Thin-film solar cells are a type of solar cell made by depositing one or more thin layers (thin films or TFs) of photovoltaic material onto a substrate, such as glass, plastic or metal. Thin-film solar cells are typically a few nanometers (nan ...
s, and other microelectronics. In these applications is initially broken down within a plasma. The resulting fluorine radicals are the active agents that attack polysilicon
Polycrystalline silicon, or multicrystalline silicon, also called polysilicon, poly-Si, or mc-Si, is a high purity, polycrystalline form of silicon, used as a raw material by the solar photovoltaic and electronics industry.
Polysilicon is produ ...
, silicon nitride and silicon oxide. They can be used as well to remove tungsten silicide, tungsten
Tungsten (also called wolfram) is a chemical element; it has symbol W and atomic number 74. It is a metal found naturally on Earth almost exclusively in compounds with other elements. It was identified as a distinct element in 1781 and first ...
, and certain other metals. In addition to serving as an etchant in device fabrication, is also widely used to clean PECVD chambers.
dissociates more readily within a low-pressure discharge in comparison to perfluorinated compound
A perfluorinated compound (PFC) or perfluoro compound is an Organofluorine chemistry, organofluorine compound that lacks C-H bonds. Many perfluorinated compounds have properties that are quite different from their C-H containing analogues. Common ...
s (PFCs) and sulfur hexafluoride (). The greater abundance of negatively-charged free radicals thus generated can yield higher silicon removal rates, and provide other process benefits such as less residual contamination and a lower net charge stress on the device being fabricated. As a somewhat more thoroughly consumed etching and cleaning agent, NF3 has also been promoted as an environmentally preferable substitute for or PFCs such as hexafluoroethane.
The utilization efficiency of the chemicals applied in plasma processes varies widely between equipment and applications. A sizeable fraction of the reactants are wasted into the exhaust stream and can ultimately be emitted into Earth's atmosphere. Modern abatement systems can substantially decrease atmospheric emissions. has not been subject to significant use restrictions. The annual reporting of production, consumption, and waste emissions by large manufacturers has been required in many industrialized countries as a response to the observed atmospheric growth and the international Kyoto Protocol
The was an international treaty which extended the 1992 United Nations Framework Convention on Climate Change (UNFCCC) that commits state parties to reduce greenhouse gas emissions, based on the scientific consensus that global warming is oc ...
.
Highly toxic fluorine gas (F2, diatomic fluorine
Fluorine is a chemical element; it has Chemical symbol, symbol F and atomic number 9. It is the lightest halogen and exists at Standard temperature and pressure, standard conditions as pale yellow Diatomic molecule, diatomic gas. Fluorine is extre ...
) is a climate neutral replacement for nitrogen trifluoride in some manufacturing applications. It requires more stringent handling and safety precautions, especially to protect manufacturing personnel.
Nitrogen trifluoride is also used in hydrogen fluoride and deuterium fluoride lasers, which are types of chemical lasers. There it is also preferred to fluorine gas due to its more convenient handling properties
Greenhouse gas
 The GWP of is second only to in the group of Kyoto-recognised greenhouse gases, and was included in that grouping with effect from 2013 and the commencement of the second commitment period of the Kyoto Protocol. It has an estimated atmospheric lifetime of 740 years, although other work suggests a slightly shorter lifetime of 550 years (and a corresponding GWP of 16,800).
The GWP of is second only to in the group of Kyoto-recognised greenhouse gases, and was included in that grouping with effect from 2013 and the commencement of the second commitment period of the Kyoto Protocol. It has an estimated atmospheric lifetime of 740 years, although other work suggests a slightly shorter lifetime of 550 years (and a corresponding GWP of 16,800).
 Since 1992, when less than 100 tons were produced, production grew to an estimated 4000 tons in 2007 and is projected to increase significantly. World production of NF3 is expected to reach 8000 tons a year by 2010. By far the world's largest producer of is the US
Since 1992, when less than 100 tons were produced, production grew to an estimated 4000 tons in 2007 and is projected to increase significantly. World production of NF3 is expected to reach 8000 tons a year by 2010. By far the world's largest producer of is the US industrial gas
Industrial gases are the gaseous materials that are Manufacturing, manufactured for use in Industrial sector, industry. The principal gases provided are nitrogen, oxygen, carbon dioxide, argon, hydrogen, helium and acetylene, although many other ...
and chemical company Air Products & Chemicals. An estimated 2% of produced is released into the atmosphere. Robson projected that the maximum atmospheric concentration is less than 0.16 parts per trillion (ppt) by volume, which will provide less than 0.001 Wm−2 of IR forcing.
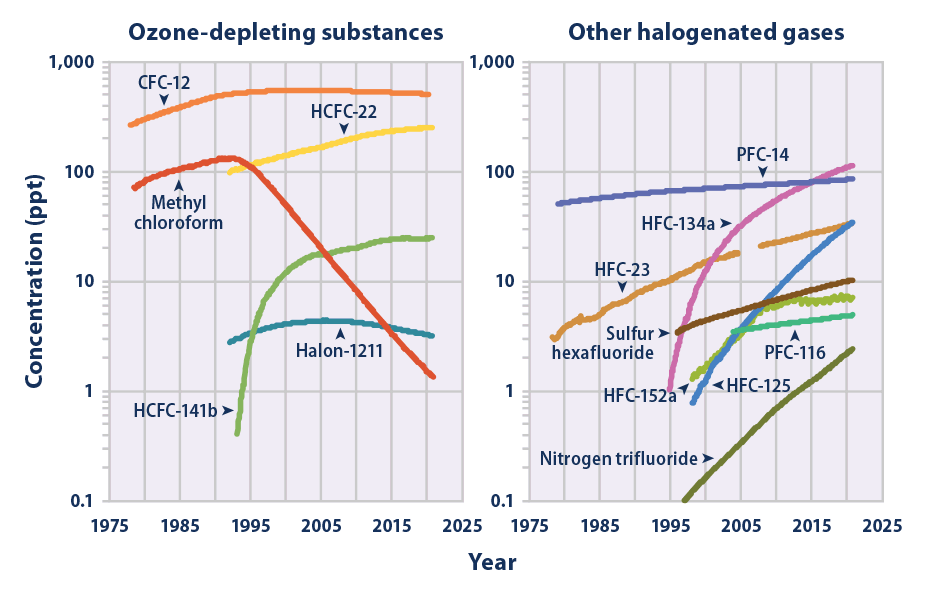
The mean global tropospheric concentration of NF3 has risen from about 0.02 ppt (parts per trillion, dry air mole fraction) in 1980, to 0.86 ppt in 2011, with a rate of increase of 0.095 ppt yr−1, or about 11% per year, and an interhemispheric gradient that is consistent with emissions occurring overwhelmingly in the Northern Hemisphere, as expected. This rise rate in 2011 corresponds to about 1200 metric tons/y NF3 emissions globally, or about 10% of the NF3 global production estimates. This is a significantly higher percentage than has been estimated by industry, and thus strengthens the case for inventorying NF3 production and for regulating its emissions.
One study co-authored by industry representatives suggests that the contribution of the NF3 emissions to the overall greenhouse gas
Greenhouse gases (GHGs) are the gases in the atmosphere that raise the surface temperature of planets such as the Earth. Unlike other gases, greenhouse gases absorb the radiations that a planet emits, resulting in the greenhouse effect. T ...
budget of thin-film Si-solar cell manufacturing is clear.
The UNFCCC, within the context of the Kyoto Protocol, decided to include nitrogen trifluoride in the second Kyoto Protocol
The was an international treaty which extended the 1992 United Nations Framework Convention on Climate Change (UNFCCC) that commits state parties to reduce greenhouse gas emissions, based on the scientific consensus that global warming is oc ...
compliance period, which begins in 2012 and ends in either 2017 or 2020. Following suit, the WBCSD/WRI GHG Protocol is amending all of its standards (corporate, product and Scope 3) to also cover NF3.
Safety
Skin contact with is not hazardous, and it is a relatively minor irritant tomucous membrane
A mucous membrane or mucosa is a membrane that lines various cavities in the body of an organism and covers the surface of internal organs. It consists of one or more layers of epithelial cells overlying a layer of loose connective tissue. It ...
s and eyes. It is a pulmonary irritant with a toxicity
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacteria, bacterium, or plant, as well as the effect o ...
considerably lower than nitrogen oxides, and overexposure via inhalation causes the conversion of hemoglobin
Hemoglobin (haemoglobin, Hb or Hgb) is a protein containing iron that facilitates the transportation of oxygen in red blood cells. Almost all vertebrates contain hemoglobin, with the sole exception of the fish family Channichthyidae. Hemoglobin ...
in blood to methemoglobin
Methemoglobin (British: methaemoglobin, shortened MetHb) (pronounced "met-hemoglobin") is a hemoglobin ''in the form of metalloprotein'', in which the iron in the heme group is in the Fe3+ (ferric) state, not the Fe2+ (ferrous) of normal hemoglobin ...
, which can lead to the condition methemoglobinemia
Methemoglobinemia, or methaemoglobinaemia, is a condition of elevated methemoglobin in the blood. Symptoms may include headache, dizziness, shortness of breath, nausea, poor muscle coordination, and blue-colored skin (cyanosis). Complications ma ...
. The National Institute for Occupational Safety and Health
The National Institute for Occupational Safety and Health (NIOSH, ) is the List of United States federal agencies, United States federal agency responsible for conducting research and making recommendations for the prevention of work-related occ ...
(NIOSH) specifies that the concentration that is immediately dangerous to life or health (IDLH value) is 1,000 ppm.
See also
* IPCC list of greenhouse gases * Nitrogen pentafluoride * TetrafluorohydrazineNotes
References
External links
*NF3
Code of Practice (European Industrial Gas Association)]
WebBook page for NF3
{{DEFAULTSORT:Nitrogen Trifluoride Inorganic amines Nitrogen fluorides Greenhouse gases Industrial gases Nitrogen(III) compounds