|
Semialdehyde
In organic chemistry, a semialdehyde is a compound containing an aldehyde and a carboxylic acid functional groups. Semialdehydes are common in biochemistry. The simplest semialdehydes have the formula . As illustrated by the behavior of the smallest member, glyoxylic acid, semialdehydes often exist as hydrates (geminal diols) . Some of semialdehydes and their parent dicarboxylic acids are listed below. {, class="wikitable" , + Selected Semialdehydes and their parent diacid , - ! Semialdehyde!! Dicarboxylic acid , - , malonic semialdehyde , malonic acid , - , tartronic semialdehyde , tartronic acid , - , succinic semialdehyde , succinic acid , - , methylmalonic semialdehyde , methylmalonic acid , - , aspartic-4-semialdehyde , aspartic acid , - , glutamate-1-semialdehyde, glutamic-1-semialdehyde , glutamic acid , - , glutamate-5-semialdehyde, glutamic-5-semialdehyde , glutamic acid , - , 4-Hydroxymuconic-semialdehyde, 4-hydroxymuconic-semialdehyde , 4-Hydroxymuconic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Malonic Semialdehyde
3-Oxopropanoic acid (also malonic semialdehyde or formylacetic acid) is an organic chemical compound that carries both a carboxylic acid function and an aldehyde function. Natural occurence In nature, 3-oxopropanoic acid occurs as a metabolic intermediate. It is formed, for example, by the reversible oxidation of 3-hydroxypropionyl-CoA with nicotinamide adenine dinucleotide (NAD). A bacterial strain of the species ''Pseudomonas fluorescens'' is known to survive on propiolic acid as its sole carbon and energy source. 3-Oxopropanoic acid is an important metabolic intermediate: it is formed by hydration of acetylenic acid and is converted into acetyl-CoA by decarboxylation. It also occurs as a metabolic intermediate in a strain of ''Escherichia coli'' that can grow on uracil as its sole nitrogen source. 3-Oxopropanoic acid also occurs in atmospheric aerosols along with various other organic acids (especially oxalic acid). It has been detected as an aerosol component at various statio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
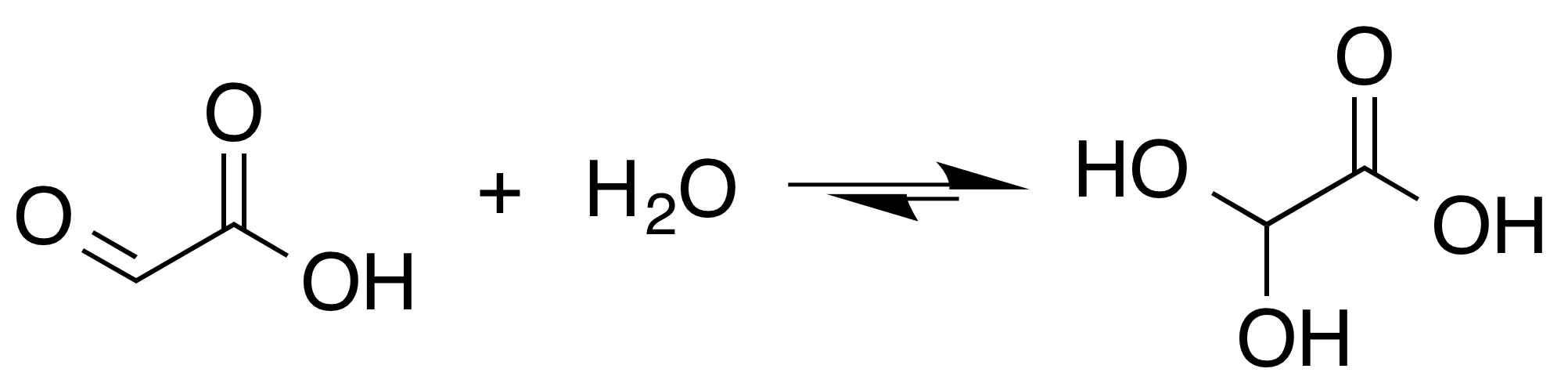
Glyoxylic Acid
Glyoxylic acid or oxoacetic acid is an organic compound. Together with acetic acid, glycolic acid, and oxalic acid, glyoxylic acid is one of the C2 carboxylic acids. It is a colourless solid that occurs naturally and is useful industrially. Structure and nomenclature The structure of glyoxylic acid is shown as having an aldehyde functional group. The aldehyde is only a minor component of the form most prevalent in some situations. Instead, glyoxylic acid often exists as a hydrate or a cyclic dimer (chemistry), dimer. For example, in the presence of water, the carbonyl rapidly converts to a geminal diol (described as the "monohydrate"). The equilibrium constant (''K'') is 300 for the formation of dihydroxyacetic acid at room temperature: Dihydroxyacetic acid has been characterized by X-ray crystallography. : In aqueous solution, this monohydrate exists in equilibrium with a hemiacylal dimer form:Georges Mattioda and Yani Christidis “Glyoxylic Acid” Ullmann's Encyclopedia of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are a common motif in many chemicals important in technology and biology. Structure and bonding Aldehyde molecules have a central carbon atom that is connected by a double bond to oxygen, a single bond to hydrogen and another single bond to a third substituent, which is carbon or, in the case of formaldehyde, hydrogen. The central carbon is often described as being sp2- hybridized. The aldehyde group is somewhat polar. The bond length is about 120–122 picometers. Physical properties and characterization Aldehydes have properties that are diverse and that depend on the remainder of the molecule. Smaller aldehydes such as formaldehyde and acetaldehyde are solubl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes Physical property, physical and Chemical property, chemical properties, and evaluation of Reactivity (chemistry), chemical reactivity to understand their behavior. The study of organic reactions includes the organic synthesis, chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical (in silico) study. The range of chemicals studied chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylmalonic Acid
Methylmalonic acid (MMA) is a chemical compound from the group of dicarboxylic acids. It consists of the basic structure of malonic acid and also carries a methyl group. The salts of methylmalonic acid are called methylmalonates. Metabolism Methylmalonic acid is a by-product of the propionate metabolism pathway. The starting sources for this are the following with the respective approximate contributions to whole body propionate metabolism in brackets: * essential amino acids: methionine, valine, threonine and isoleucine (~ 50%) * odd-chain fatty acids (~ 30%) * propionic acid from bacterial fermentation (~ 20%) * cholesterol side chain * thymine The propionate derivative, propionyl-CoA, is converted into D- methylmalonyl-CoA by propionyl-CoA carboxylase and then converted into L-methylmalonyl-CoA by methylmalonyl-CoA epimerase. Entry into the citric acid cycle occurs through the conversion of L-methylmalonyl-CoA into succinyl-CoA by L-methylmalonyl-CoA mutase, whereby vi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allysine
Allysine is a derivative of lysine that features a formyl group in place of the terminal amine. The free amino acid does not exist, but the allysine ''residue'' does. It is produced by aerobic oxidation of lysine residues by the enzyme lysyl oxidase. The transformation is an example of a post-translational modification. The semialdehyde form exists in equilibrium with a cyclic derivative. Allysine is involved in the production of elastin and collagen. Increased allysine concentration in tissues has been correlated to the presence of fibrosis. Allysine residues react with sodium 2-naphthol-6-sulfonate to produce a fluorescent Fluorescence is one of two kinds of photoluminescence, the emission of light by a substance that has absorbed light or other electromagnetic radiation. When exposed to ultraviolet radiation, many substances will glow (fluoresce) with color ... bis-naphtol-allysine product. In another assay, allysine-containing proteins are reduced with sodiu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glutamate-5-semialdehyde
Glutamate-5-semialdehyde is a non-proteinogenic amino acid involved in both the biosynthesis and degradation of proline and arginine (via ornithine), as well as in the biosynthesis of antibiotics, such as carbapenems. It is synthesized by the reduction of glutamyl-5-phosphate by glutamate-5-semialdehyde dehydrogenase. Reduction of glutamic acid semialdehyde with sodium borohydride Sodium borohydride, also known as sodium tetrahydridoborate and sodium tetrahydroborate, is an inorganic compound with the formula (sometimes written as ). It is a white crystalline solid, usually encountered as an aqueous basic solution. Sodi ... gives hydroxyaminovaleric acid. See also * Glutamate-1-semialdehyde References {{Amino acid metabolism intermediates Alpha-Amino acids Aldehydic acids ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glutamic Acid
Glutamic acid (symbol Glu or E; known as glutamate in its anionic form) is an α- amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a non-essential nutrient for humans, meaning that the human body can synthesize enough for its use. It is also the most abundant excitatory neurotransmitter in the vertebrate nervous system. It serves as the precursor for the synthesis of the inhibitory gamma-aminobutyric acid (GABA) in GABAergic neurons. Its molecular formula is . Glutamic acid exists in two optically isomeric forms; the dextrorotatory -form is usually obtained by hydrolysis of gluten or from the waste waters of beet-sugar manufacture or by fermentation.Webster's Third New International Dictionary of the English Language Unabridged, Third Edition, 1971. Its molecular structure could be idealized as HOOC−CH()−()2−COOH, with two carboxyl groups −COOH and one amino group −. However, in the solid state and mildly acidic water s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glutamate-1-semialdehyde
Glutamate-1-semialdehyde is a molecule formed from by the reduction of tRNA bound glutamate, catalyzed by glutamyl-tRNA reductase. It is isomerized by glutamate-1-semialdehyde 2,1-aminomutase to give aminolevulinic acid in the biosynthesis of porphyrins, including heme and chlorophyll. See also * Glutamate-5-semialdehyde Glutamate-5-semialdehyde is a non-proteinogenic amino acid involved in both the biosynthesis and degradation of proline and arginine (via ornithine), as well as in the biosynthesis of antibiotics, such as carbapenems. It is synthesized by the redu ... References {{biochem-stub Aldehydes Gamma-Amino acids ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |