|
Methylmalonic Acid
Methylmalonic acid (MMA) is a chemical compound from the group of dicarboxylic acids. It consists of the basic structure of malonic acid and also carries a methyl group. The salts of methylmalonic acid are called methylmalonates. Metabolism Methylmalonic acid is a by-product of the propionate metabolism pathway. The starting sources for this are the following with the respective approximate contributions to whole body propionate metabolism in brackets: * essential amino acids: methionine, valine, threonine and isoleucine (~ 50%) * odd-chain fatty acids (~ 30%) * propionic acid from bacterial fermentation (~ 20%) * cholesterol side chain * thymine The propionate derivative, propionyl-CoA, is converted into D- methylmalonyl-CoA by propionyl-CoA carboxylase and then converted into L-methylmalonyl-CoA by methylmalonyl-CoA epimerase. Entry into the citric acid cycle occurs through the conversion of L-methylmalonyl-CoA into succinyl-CoA by L-methylmalonyl-CoA mutase, whereby vi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound. A compound can be transformed into a different substance by a chemical reaction, which may involve interactions with other substances. In this process, bonds between atoms may be broken or new bonds formed or both. There are four major types of compounds, distinguished by how the constituent atoms are bonded together. Molecular compounds are held together by covalent bonds; ionic compounds are held together by ionic bonds; intermetallic compounds are held together by metallic bonds; coordination complexes are held together by coordinate covalent bonds. Non-stoichiometric compounds form a disputed marginal case. A chemical formula specifies the number of atoms of each element in a compound molecule, usin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propionyl-CoA
Propionyl-CoA is a coenzyme A derivative of propionic acid. It is composed of a 24 total carbon chain (without the coenzyme, it is a 3 carbon structure) and its production and metabolic fate depend on which organism it is present in. Several different pathways can lead to its production, such as through the catabolism of specific amino acids or the oxidation of odd-chain fatty acids. It later can be broken down by propionyl-CoA carboxylase or through the methylcitrate cycle. In different organisms, however, propionyl-CoA can be sequestered into controlled regions, to alleviate its potential toxicity through accumulation. Genetic deficiencies regarding the production and breakdown of propionyl-CoA also have great clinical and human significance. Production There are several different pathways through which propionyl-CoA can be produced: * Propionyl-CoA, a three-carbon structure, is considered to be a minor species of propionic acid. Therefore, odd-number chains of fatty acids a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
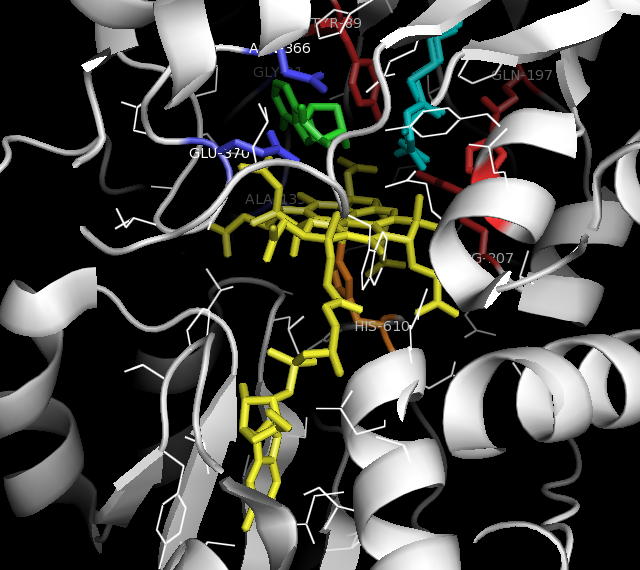
ACSF3
Acyl-CoA synthetase family member 3 is an enzyme that in humans is encoded by the ''ACSF3'' gene. The enzyme belongs to the Acyl-CoA synthetase, acyl-CoA synthetase family. Structure The ''ACSF3'' gene is located on the Chromosome 16, 16th chromosome, with its specific location being 16q24.3. The gene contains 17 Exon, exons. ''ACSF3'' encodes a 64.1 kDa protein that is composed of 576 Amino acid, amino acids; 20 Peptide, peptides have been observed through mass spectrometry data. Function This gene encodes a member of the Acyl-CoA synthetase, acyl-CoA synthetase family of enzymes that activate fatty acids by catalyzing the formation of a thioester linkage between Fatty acid, fatty acids and coenzyme A. The encoded protein is localized to mitochondria, has high specificity for Malonic acid, malonate and Methylmalonic acid, methylmalonate and possesses Malonate—CoA ligase, malonyl-CoA synthetase activity: :Adenosine triphosphate, ATP + Malonic acid, malonate + Coenzyme A, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
(S)-methylmalonyl-CoA Hydrolase
The enzyme (''S'')-methylmalonyl-CoA hydrolase (EC 3.1.2.17) catalyzes the reaction :(''S'')-methylmalonyl-CoA + HO \rightleftharpoons methylmalonate + CoA This enzyme belongs to the family of hydrolases, specifically those acting on thioester bonds. The systematic name of this enzyme class is (''S'')-methylmalonyl-CoA hydrolase. This enzyme is also called D-methylmalonyl-coenzyme A hydrolase. This enzyme participates in propanoate metabolism Propionic acid (, from the Greek words πρῶτος : ''prōtos'', meaning "first", and πίων : ''píōn'', meaning "fat"; also known as propanoic acid) is a naturally occurring carboxylic acid with chemical formula . It is a liquid with a p .... References * EC 3.1.2 Enzymes of unknown structure {{3.1-enzyme-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coenzyme A
Coenzyme A (CoA, SHCoA, CoASH) is a coenzyme, notable for its role in the Fatty acid metabolism#Synthesis, synthesis and Fatty acid metabolism#.CE.B2-Oxidation, oxidation of fatty acids, and the oxidation of pyruvic acid, pyruvate in the citric acid cycle. All genomes sequenced to date encode enzymes that use coenzyme A as a Substrate (chemistry), substrate, and around 4% of cellular enzymes use it (or a thioester) as a substrate. In humans, CoA biosynthesis requires cysteine, pantothenic acid, pantothenate (vitamin B5), and adenosine triphosphate (ATP). In acetyl-CoA, its acetyl form, coenzyme A is a highly versatile molecule, serving metabolic functions in both the Anabolism, anabolic and Catabolism, catabolic pathways. Acetyl-CoA is utilised in the post-translational regulation and allosteric regulation of pyruvate dehydrogenase and carboxylase to maintain and support the partition of Pyruvic acid, pyruvate synthesis and degradation. Discovery of structure Coenzyme A was ident ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anaplerotic Reactions
Anaplerotic reactions, a term coined by Hans Kornberg and originating from the Greeἀνά 'up' anπληρόω 'to fill', are chemical reactions that form intermediates of a metabolic pathway. Examples of such are found in the citric acid cycle (TCA cycle). In normal function of this cycle for respiration, concentrations of TCA intermediates remain constant; however, many biosynthetic reactions also use these molecules as a substrate. Anaplerosis is the act of replenishing TCA cycle intermediates that have been extracted for biosynthesis (in what are called anaplerotic reactions). The TCA cycle is a hub of metabolism, with central importance in both energy production and biosynthesis. Therefore, it is crucial for the cell to regulate concentrations of TCA cycle metabolites in the mitochondria. Anaplerotic flux must balance cataplerotic flux in order to retain homeostasis of cellular metabolism. Reactions of anaplerotic metabolism There are five major reactions classed as anaple ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coenzyme
A cofactor is a non-protein chemical compound or Metal ions in aqueous solution, metallic ion that is required for an enzyme's role as a catalysis, catalyst (a catalyst is a substance that increases the rate of a chemical reaction). Cofactors can be considered "helper molecules" that assist in Biochemistry, biochemical transformations. The rates at which these happen are characterized in an area of study called enzyme kinetics. Cofactors typically differ from Ligand (biochemistry), ligands in that they often derive their function by remaining bound. Cofactors can be classified into two types: inorganic ions and complex organic molecules called Enzyme#Coenzymes, coenzymes. Coenzymes are mostly derived from vitamins and other organic essential nutrients in small amounts. (Some scientists limit the use of the term "cofactor" for inorganic substances; both types are included here.) Coenzymes are further divided into two types. The first is called a "prosthetic group", which consists ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adenosylcobalamin
Adenosylcobalamin (AdoCbl), also known as coenzyme B12, cobamamide, and dibencozide, is one of the biologically active forms of vitamin B12. Adenosylcobalamin participates as a cofactor in radical-mediated 1,2-carbon skeleton rearrangements. These processes require the formation of the deoxyadenosyl radical through homolytic dissociation of the carbon-cobalt bond. This bond is exceptionally weak, with a bond dissociation energy of 31 kcal/mol, which is further lowered in the chemical environment of an enzyme active site. An enzyme that uses adenosylcobalamin as a coenzyme is methylmalonyl-CoA mutase (MCM). Further experimentation has also determined adenosylcobalamin's role in regulating expression of some bacterial genes. By binding to CarH, AdoCbl can modulate carotenoid genes, which confer warm colors onto various plants. Carotenoid transcription is activated by sunlight, due to the response from AdoCbl. There are other photoreceptors across different bacterial communities, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vitamin B12
Vitamin B12, also known as cobalamin, is a water-soluble vitamin involved in metabolism. One of eight B vitamins, it serves as a vital cofactor (biochemistry), cofactor in DNA synthesis and both fatty acid metabolism, fatty acid and amino acid metabolism. It plays an essential role in the nervous system by supporting myelinogenesis, myelin synthesis and is critical for the maturation of red blood cells in the bone marrow. While animals require B12, plants do not, relying instead on alternative enzymatic pathways. Vitamin B12 is the most chemically complex of all vitamins, and is synthesized exclusively by certain archaea and bacteria. Natural food sources include meat, shellfish, liver, fish, poultry, Egg as food, eggs, and dairy products. It is also added to many breakfast cereals through food fortification and is available in dietary supplement and pharmaceutical forms. Supplements are commonly taken orally but may be administered via intramuscular injection to treat defic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylmalonyl-CoA Mutase
Methylmalonyl-CoA mutase (, MCM), mitochondrial, also known as methylmalonyl-CoA isomerase, is a protein that in humans is encoded by the ''MUT'' gene. This vitamin B12-dependent enzyme catalyzes the isomerization of methylmalonyl-CoA to succinyl-CoA in humans. Mutations in ''MUT'' gene may lead to various types of methylmalonic aciduria. MCM was first identified in rat liver and sheep kidney in 1955. In its latent form, it is 750 amino acids in length. Upon entry to the mitochondria, the 32 amino acid mitochondrial leader sequence at the N-terminus of the protein is cleaved, forming the fully processed monomer. The monomers then associate into homodimers, and bind AdoCbl (one for each monomer active site) to form the final, active holoenzyme form. Structure Gene The ''MUT'' gene lies on the chromosome location of 6p12.3 and consists of 13 exons, spanning over 35kb. Protein The mature enzyme is a homodimer with the N-terminal CoA binding domain and the C- terminal cobala ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Succinyl-CoA
Succinyl-coenzyme A, abbreviated as succinyl-CoA () or SucCoA, is a thioester of succinic acid and coenzyme A. Sources It is an important intermediate in the citric acid cycle, where it is synthesized from Alpha-Ketoglutaric acid, α-ketoglutarate by Alpha-ketoglutarate dehydrogenase, α-ketoglutarate dehydrogenase through decarboxylation. During the process, coenzyme A is added. With B12 as an enzymatic cofactor, it is also synthesized from propionyl coenzymeA, propionyl CoA, the odd-numbered fatty acid, which cannot undergo beta-oxidation. Propionyl-CoA is carboxylated to D-methylmalonyl-CoA, isomerized to L-methylmalonyl-CoA, and rearranged to yield succinyl-CoA via a vitamin B12, vitamin B12-dependent enzyme. While Succinyl-CoA is an intermediate of the citric acid cycle, it cannot be readily incorporated there because there is no net consumption of Succinyl-CoA. Succinyl-CoA is first converted to malate, and then to pyruvate where it is then transported to the matrix to enter ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Citric Acid Cycle
The citric acid cycle—also known as the Krebs cycle, Szent–Györgyi–Krebs cycle, or TCA cycle (tricarboxylic acid cycle)—is a series of chemical reaction, biochemical reactions that release the energy stored in nutrients through acetyl-CoA Redox, oxidation. The energy released is available in the form of Adenosine triphosphate, ATP. The Hans Krebs (biochemist), Krebs cycle is used by organisms that generate energy via Cellular respiration, respiration, either anaerobic respiration, anaerobically or aerobic respiration, aerobically (organisms that Fermentation, ferment use different pathways). In addition, the cycle provides precursor (chemistry), precursors of certain amino acids, as well as the reducing agent nicotinamide adenine dinucleotide, NADH, which are used in other reactions. Its central importance to many Metabolic pathway, biochemical pathways suggests that it was one of the earliest metabolism components. Even though it is branded as a "cycle", it is not necessa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |