Glyoxylic Acid on:
[Wikipedia]
[Google]
[Amazon]
Glyoxylic acid or oxoacetic acid is an
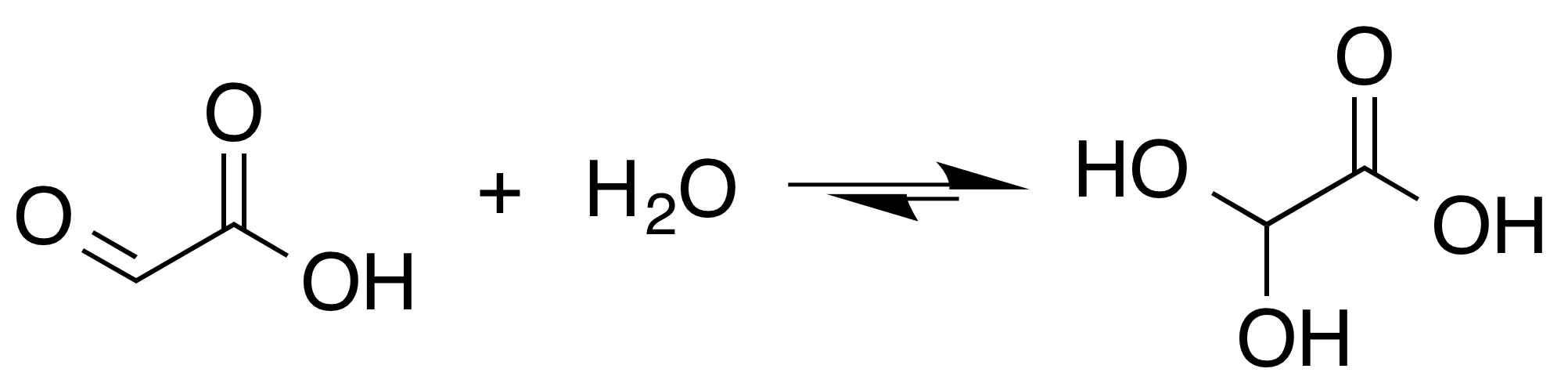 In aqueous solution, this monohydrate exists in equilibrium with a hemi acylal dimer form:Georges Mattioda and Yani Christidis “Glyoxylic Acid” Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim.
:
In aqueous solution, this monohydrate exists in equilibrium with a hemi acylal dimer form:Georges Mattioda and Yani Christidis “Glyoxylic Acid” Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim.
: In isolation, the aldehyde structure has as a major
In isolation, the aldehyde structure has as a major 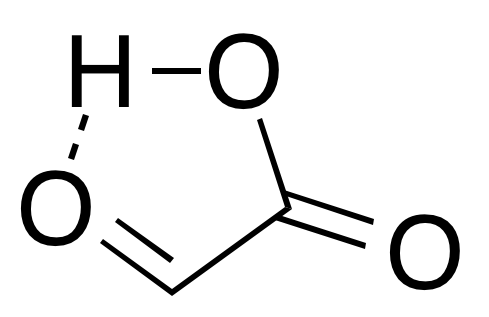 The
The


organic compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-co ...
. Together with acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main compone ...
, glycolic acid
Glycolic acid (or hydroxyacetic acid; chemical formula ) is a colorless, odorless and hygroscopic crystal, crystalline solid, highly solubility, soluble in water. It is used in various skin care, skin-care products. Glycolic acid is widespread in ...
, and oxalic acid
Oxalic acid is an organic acid with the systematic name ethanedioic acid and chemical formula , also written as or or . It is the simplest dicarboxylic acid. It is a white crystalline solid that forms a colorless solution in water. Its name i ...
, glyoxylic acid is one of the C2 carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an Substituent, R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl ...
s. It is a colourless solid that occurs naturally and is useful industrially.
Structure and nomenclature
The structure of glyoxylic acid is shown as having analdehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
functional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
. The aldehyde is only a minor component of the form most prevalent in some situations. Instead, glyoxylic acid often exists as a hydrate or a cyclic dimer. For example, in the presence of water, the carbonyl
In organic chemistry, a carbonyl group is a functional group with the formula , composed of a carbon atom double bond, double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds (such a ...
rapidly converts to a geminal diol
A geminal diol (or gem-diol for short) is any organic compound having two hydroxyl functional groups () bound to the same carbon atom. Geminal diols are a subclass of the diols, which in turn are a special class of alcohols. Most of the geminal ...
(described as the "monohydrate"). The equilibrium constant
The equilibrium constant of a chemical reaction is the value of its reaction quotient at chemical equilibrium, a state approached by a dynamic chemical system after sufficient time has elapsed at which its composition has no measurable tendency ...
(''K'') is 300 for the formation of dihydroxyacetic acid at room temperature: Dihydroxyacetic acid has been characterized by X-ray crystallography
X-ray crystallography is the experimental science of determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to Diffraction, diffract in specific directions. By measuring th ...
.
: In aqueous solution, this monohydrate exists in equilibrium with a hemi acylal dimer form:Georges Mattioda and Yani Christidis “Glyoxylic Acid” Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim.
:
In aqueous solution, this monohydrate exists in equilibrium with a hemi acylal dimer form:Georges Mattioda and Yani Christidis “Glyoxylic Acid” Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim.
: In isolation, the aldehyde structure has as a major
In isolation, the aldehyde structure has as a major conformer
A conformer is a clear acrylic shell fitted after an enucleation of the eye
Enucleation is the removal of the eye that leaves the eye muscles and remaining orbital contents intact. This type of ocular surgery is indicated for a number of oc ...
a cyclic hydrogen-bond
In chemistry, a hydrogen bond (H-bond) is a specific type of molecular interaction that exhibits partial covalent character and cannot be described as a purely electrostatic force. It occurs when a hydrogen (H) atom, covalently bonded to a mo ...
ed structure with the aldehyde carbonyl in close proximity to the carboxyl
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl group (e.g. ...
hydrogen:
: The
The Henry's law
In physical chemistry, Henry's law is a gas law that states that the amount of dissolved gas in a liquid is directly proportional at equilibrium to its partial pressure above the liquid. The proportionality factor is called Henry's law constant ...
constant of glyoxylic acid is KH = 1.09 × 104 × exp 40.0 × 103/R) × (1/T − 1/298)
Preparations
Theconjugate base
A conjugate acid, within the Brønsted–Lowry acid–base theory, is a chemical compound formed when an acid gives a proton () to a base—in other words, it is a base with a hydrogen ion added to it, as it loses a hydrogen ion in the reve ...
of glyoxylic acid is known as glyoxylate and is the form that the compound exists in solution at neutral pH. Glyoxylate is the byproduct of the amidation
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent any group, typically organyl groups or hydrogen atoms. The amide group is called a p ...
process in biosynthesis of several amidated peptide
Peptides are short chains of amino acids linked by peptide bonds. A polypeptide is a longer, continuous, unbranched peptide chain. Polypeptides that have a molecular mass of 10,000 Da or more are called proteins. Chains of fewer than twenty am ...
s.
For the historical record, glyoxylic acid was prepared from oxalic acid electrosynthetically: in organic synthesis, lead
Lead () is a chemical element; it has Chemical symbol, symbol Pb (from Latin ) and atomic number 82. It is a Heavy metal (elements), heavy metal that is density, denser than most common materials. Lead is Mohs scale, soft and Ductility, malleabl ...
cathodes were applied for preparing glyoxylic acid from oxalic acid
Oxalic acid is an organic acid with the systematic name ethanedioic acid and chemical formula , also written as or or . It is the simplest dicarboxylic acid. It is a white crystalline solid that forms a colorless solution in water. Its name i ...
in a sulfuric acid electrolyte.
: 380px
Hot nitric acid
Nitric acid is an inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into nitrogen oxide, oxides of nitrogen. Most com ...
can oxidize
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
glyoxal
Glyoxal is an organic compound with the chemical formula OCHCHO. It is the smallest dialdehyde (a compound with two aldehyde groups). It is a crystalline solid, white at low temperatures and yellow near the melting point (15 °C). The liquid ...
to glyoxylic acid, however this reaction is highly exothermic and prone to thermal runaway. In addition, oxalic acid is the main side product.
Also, ozonolysis
In organic chemistry, ozonolysis is an organic reaction where the Saturated and unsaturated compounds, unsaturated bonds are Bond cleavage, cleaved with ozone (). Multiple carbon–carbon bond are replaced by carbonyl () groups, such as aldehydes ...
of maleic acid
Maleic acid or ''cis''-butenedioic acid is an organic compound that is a dicarboxylic acid, a molecule with two carboxyl groups. Its chemical formula is HO2CCH=CHCO2H. Maleic acid is the ''cis'' Cis–trans isomerism, isomer of butenedioic acid, ...
is effective.
Biological role
Glyoxylate is an intermediate of theglyoxylate cycle
The glyoxylate cycle, a variation of the tricarboxylic acid cycle, is an anabolic pathway occurring in plants, bacteria, protists, and fungi. The glyoxylate cycle centers on the conversion of acetyl-CoA to succinate for the synthesis of carbohy ...
, which enables organism
An organism is any life, living thing that functions as an individual. Such a definition raises more problems than it solves, not least because the concept of an individual is also difficult. Many criteria, few of them widely accepted, have be ...
s, such as bacteria, fungi, and plants to convert fatty acid
In chemistry, in particular in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated and unsaturated compounds#Organic chemistry, saturated or unsaturated. Most naturally occurring fatty acids have an ...
s into carbohydrate
A carbohydrate () is a biomolecule composed of carbon (C), hydrogen (H), and oxygen (O) atoms. The typical hydrogen-to-oxygen atomic ratio is 2:1, analogous to that of water, and is represented by the empirical formula (where ''m'' and ''n'' ...
s. The glyoxylate cycle is also important for induction of plant defense mechanisms in response to fungi. The glyoxylate cycle is initiated through the activity of isocitrate lyase, which converts isocitrate into glyoxylate and succinate. Research is being done to co-opt the pathway for a variety of uses such as the biosynthesis of succinate.
In humans
Glyoxylate is produced via two pathways: through the oxidation of glycolate in peroxisomes or through the catabolism of hydroxyproline in mitochondria. In the peroxisomes, glyoxylate is converted into glycine by AGT1 or into oxalate by glycolate oxidase. In the mitochondria, glyoxylate is converted into glycine by AGT2 or into glycolate by glyoxylate reductase. A small amount of glyoxylate is converted into oxalate by cytoplasmic lactate dehydrogenase.
In plants
In addition to being an intermediate in theglyoxylate cycle
The glyoxylate cycle, a variation of the tricarboxylic acid cycle, is an anabolic pathway occurring in plants, bacteria, protists, and fungi. The glyoxylate cycle centers on the conversion of acetyl-CoA to succinate for the synthesis of carbohy ...
, glyoxylate is also an important intermediate in the photorespiration
Photorespiration (also known as the oxidative photosynthetic carbon cycle or C2 cycle) refers to a process in plant physiology, plant metabolism where the enzyme RuBisCO oxygenates RuBP, wasting some of the energy produced by photosynthesis. Th ...
pathway. Photorespiration is a result of the side reaction of RuBisCO with O2 instead of CO2. While at first considered a waste of energy and resources, photorespiration has been shown to be an important method of regenerating carbon and CO2, removing toxic phosphoglycolate, and initiating defense mechanisms. In photorespiration, glyoxylate is converted from glycolate through the activity of glycolate oxidase in the peroxisome. It is then converted into glycine through parallel actions by SGAT and GGAT, which is then transported into the mitochondria. It has also been reported that the pyruvate dehydrogenase complex may play a role in glycolate and glyoxylate metabolism.

Disease relevance
Diabetes
Glyoxylate is thought to be a potential early marker forType II diabetes
Type 2 diabetes (T2D), formerly known as adult-onset diabetes, is a form of diabetes mellitus that is characterized by high blood sugar, insulin resistance, and relative lack of insulin. Common symptoms include increased thirst, frequent ...
. One of the key conditions of diabetes pathology is the production of advanced glycation end-product
Advanced glycation end-products (AGEs) are proteins or lipids that become Glycation, glycated as a result of exposure to sugars. They are a bio-marker implicated in aging and the development, or worsening, of many degenerative diseases, such as dia ...
s (AGEs) caused by the hyperglycemia
Hyperglycemia is a condition where unusually high amount of glucose is present in blood. It is defined as blood glucose level exceeding 6.9 mmol/L (125 mg/dL) after fasting for 8 hours or 10 mmol/L (180 mg/dL) 2 hours after eating.
Blood gluc ...
. AGEs can lead to further complications of diabetes, such as tissue damage and cardiovascular disease. They are generally formed from reactive aldehydes, such as those present on reducing sugars and alpha-oxoaldehydes. In a study, glyoxylate levels were found to be significantly increased in patients who were later diagnosed with Type II diabetes. The elevated levels were found sometimes up to three years before the diagnosis, demonstrating the potential role for glyoxylate to be an early predictive marker.
Nephrolithiasis
Glyoxylate is involved in the development ofhyperoxaluria
Hyperoxaluria is an excessive urinary excretion of oxalate. Individuals with hyperoxaluria often have calcium oxalate kidney stones. It is sometimes called Bird's disease, after Golding Bird, who first described the condition.
Presentation
Caus ...
, a key cause of nephrolithiasis (commonly known as kidney stones
Kidney stone disease (known as nephrolithiasis, renal calculus disease, or urolithiasis) is a crystallopathy and occurs when there are too many minerals in the urine and not enough liquid or hydration. This imbalance causes tiny pieces of cr ...
). Glyoxylate is both a substrate and inductor of sulfate anion transporter-1 (sat-1), a gene
In biology, the word gene has two meanings. The Mendelian gene is a basic unit of heredity. The molecular gene is a sequence of nucleotides in DNA that is transcribed to produce a functional RNA. There are two types of molecular genes: protei ...
responsible for oxalate
Oxalate (systematic IUPAC name: ethanedioate) is an anion with the chemical formula . This dianion is colorless. It occurs naturally, including in some foods. It forms a variety of salts, for example sodium oxalate (), and several esters such as ...
transportation, allowing it to increase sat-1 mRNA
In molecular biology, messenger ribonucleic acid (mRNA) is a single-stranded molecule of RNA that corresponds to the genetic sequence of a gene, and is read by a ribosome in the process of Protein biosynthesis, synthesizing a protein.
mRNA is ...
expression and as a result oxalate efflux from the cell. The increased oxalate release allows the buildup of calcium oxalate
Calcium oxalate (in archaic terminology, oxalate of lime) is a calcium salt of oxalic acid with the chemical formula or . It forms hydrates , where ''n'' varies from 1 to 3. Anhydrous and all hydrated forms are colorless or white. The monohydr ...
in the urine
Urine is a liquid by-product of metabolism in humans and many other animals. In placental mammals, urine flows from the Kidney (vertebrates), kidneys through the ureters to the urinary bladder and exits the urethra through the penile meatus (mal ...
, and thus the eventual formation of kidney stones.
The disruption of glyoxylate metabolism
Metabolism (, from ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run cellular processes; the co ...
provides an additional mechanism of hyperoxaluria development. Loss of function mutations in the HOGA1 gene leads to a loss of the 4-hydroxy-2-oxoglutarate aldolase, an enzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different mol ...
in the hydroxyproline
(2''S'',4''R'')-4-Hydroxyproline, or L-hydroxyproline ( C5 H9 O3 N), is an amino acid, abbreviated as Hyp or O, ''e.g.'', in Protein Data Bank.
Structure and discovery
In 1902, Hermann Emil Fischer isolated hydroxyproline from hydrolyzed gela ...
to glyoxylate pathway. The glyoxylate resulting from this pathway is normally stored away to prevent oxidation
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
to oxalate in the cytosol
The cytosol, also known as cytoplasmic matrix or groundplasm, is one of the liquids found inside cells ( intracellular fluid (ICF)). It is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondri ...
. The disrupted pathway, however, causes a buildup of 4-hydroxy-2-oxoglutarate which can also be transported to the cytosol and converted into glyoxylate through a different aldolase
Fructose-bisphosphate aldolase (), often just aldolase, is an enzyme catalyzing a reversible reaction that splits the aldol, fructose 1,6-bisphosphate, into the triose phosphates dihydroxyacetone phosphate (DHAP) and glyceraldehyde 3-phosphat ...
. These glyoxylate molecules can be oxidized into oxalate increasing its concentration and causing hyperoxaluria.
Reactions and uses
Glyoxylic acid is about ten times stronger an acid thanacetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main compone ...
, with an acid dissociation constant
In chemistry, an acid dissociation constant (also known as acidity constant, or acid-ionization constant; denoted ) is a quantitative property, quantitative measure of the acid strength, strength of an acid in Solution (chemistry), solution. I ...
of 4.7 × 10−4 (p''K''a = 3.32):
:OCHCO2H + H+
Heated glyoxylic acid disproportionates in a Cannizzaro reaction
The Cannizzaro reaction, named after its discoverer Stanislao Cannizzaro, is a chemical reaction which involves the base-induced disproportionation of two molecules of a non-enolizable aldehyde to give a primary alcohol and a carboxylic acid.
...
, forming hydroxyacetic acid and oxalic acid
Oxalic acid is an organic acid with the systematic name ethanedioic acid and chemical formula , also written as or or . It is the simplest dicarboxylic acid. It is a white crystalline solid that forms a colorless solution in water. Its name i ...
:
:2 OCHCO2H + H2O → HOCH2CO2H + HO2CCO2H
Glyoxylic acid gives heterocycles upon condensation
Condensation is the change of the state of matter from the gas phase into the liquid phase, and is the reverse of vaporization. The word most often refers to the water cycle. It can also be defined as the change in the state of water vapor ...
with urea
Urea, also called carbamide (because it is a diamide of carbonic acid), is an organic compound with chemical formula . This amide has two Amine, amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest am ...
and 1,2-diaminobenzene
''o''-Phenylenediamine (OPD) is an organic compound with the formula C6H4(NH2)2. This aromatic diamine is an important precursor to many heterocyclic compounds. OPD is a white compound although samples appear darker owing to oxidation by air. I ...
.
Gloxylate ester
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distin ...
s polymerize in base, forming a poly-methyleneoxy backbone with pendant ester groups.
Phenol derivatives
In general, glyoxylic acid undergoes anelectrophilic aromatic substitution
Electrophilic aromatic substitution (SEAr) is an organic reaction in which an atom that is attached to an aromatic ring, aromatic system (usually hydrogen) is replaced by an electrophile. Some of the most important electrophilic aromatic substitut ...
reaction with phenols
In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of one or more hydroxyl groups (− O H) bonded directly to an aromatic hydrocarbon group. The simplest is phenol, . Phenolic compounds ar ...
, a versatile step in the synthesis of several other compounds.
The immediate product with phenol
Phenol (also known as carbolic acid, phenolic acid, or benzenol) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile and can catch fire.
The molecule consists of a phenyl group () ...
itself is 4-hydroxymandelic acid. This species reacts with ammonia to give hydroxyphenylglycine, a precursor to the drug amoxicillin. Reduction of the 4-hydroxymandelic acid gives 4-hydroxyphenylacetic acid, a precursor to the drug atenolol
Atenolol is a beta blocker medication primarily used to treat high blood pressure and angina, heart-associated chest pain. Although used to treat high blood pressure, it does not seem to improve mortality rate, mortality in those with the condi ...
.
The sequence of reactions, in which glyoxylic acid reacts with guaiacol
Guaiacol () is an organic compound with the formula C6H4(OH)(OCH3). It is a phenolic compound containing a methoxy functional group. Guaiacol appears as a viscous colorless oil, although aged or impure samples are often yellowish. It occurs wid ...
the phenolic component followed by oxidation and decarboxylation
Decarboxylation is a chemical reaction that removes a carboxyl group and releases carbon dioxide (CO2). Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is ...
, provides a route to vanillin
Vanillin is an organic compound with the molecular formula . It is a phenolic aldehyde. Its functional groups include aldehyde, hydroxyl, and ether. It is the primary component of the ethanolic extract of the vanilla bean. Synthetic vanillin ...
as a net formylation process.
Hopkins Cole reaction
Glyoxylic acid is a component of theHopkins–Cole reaction
The Hopkins-Cole reaction, also known as the glyoxylic acid reaction, is a chemical test used for detecting the presence of tryptophan in proteins. A protein solution is mixed with Hopkins Cole reagent, which consists of glyoxylic acid. Concentrate ...
, used to check for the presence of tryptophan
Tryptophan (symbol Trp or W)
is an α-amino acid that is used in the biosynthesis of proteins. Tryptophan contains an α-amino group, an α-carboxylic acid group, and a side chain indole, making it a polar molecule with a non-polar aromat ...
in proteins.
Hair-strengthening cosmetics
Glyoxylic acid enters the composition of cosmetic creams used for “Brazilian” hair-straightening treatment. Glyoxylic acid is used in cosmetic products in replacement offormaldehyde
Formaldehyde ( , ) (systematic name methanal) is an organic compound with the chemical formula and structure , more precisely . The compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde. It is stored as ...
to avoid skin irritation by this latter. Since the wider use of these products several persons developed acute kidney disease
Kidney disease, or renal disease, technically referred to as nephropathy, is damage to or disease of a kidney. Nephritis is an Inflammation, inflammatory kidney disease and has several types according to the location of the inflammation. Infla ...
induced by the crystallisation of calcium oxalate
Calcium oxalate (in archaic terminology, oxalate of lime) is a calcium salt of oxalic acid with the chemical formula or . It forms hydrates , where ''n'' varies from 1 to 3. Anhydrous and all hydrated forms are colorless or white. The monohydr ...
in their kidney
In humans, the kidneys are two reddish-brown bean-shaped blood-filtering organ (anatomy), organs that are a multilobar, multipapillary form of mammalian kidneys, usually without signs of external lobulation. They are located on the left and rig ...
s. Toxicity studies on mice have further demonstrated that the transcutaneous absorption of glyoxylic acid after topical application causes the excretion
Excretion is elimination of metabolic waste, which is an essential process in all organisms. In vertebrates, this is primarily carried out by the lungs, Kidney (vertebrates), kidneys, and skin. This is in contrast with secretion, where the substa ...
of oxalate
Oxalate (systematic IUPAC name: ethanedioate) is an anion with the chemical formula . This dianion is colorless. It occurs naturally, including in some foods. It forms a variety of salts, for example sodium oxalate (), and several esters such as ...
in the urine
Urine is a liquid by-product of metabolism in humans and many other animals. In placental mammals, urine flows from the Kidney (vertebrates), kidneys through the ureters to the urinary bladder and exits the urethra through the penile meatus (mal ...
at a much higher level than glycolic acid
Glycolic acid (or hydroxyacetic acid; chemical formula ) is a colorless, odorless and hygroscopic crystal, crystalline solid, highly solubility, soluble in water. It is used in various skin care, skin-care products. Glycolic acid is widespread in ...
.
Environmental chemistry
Glyoxylic acid is one of several ketone- and aldehyde-containing carboxylic acids that together are abundant in secondary organic aerosols. In the presence of water and sunlight, glyoxylic acid can undergo photochemical oxidation. Several different reaction pathways can ensue, leading to various other carboxylic acid and aldehyde products.Safety
For a long time, the compound was not considered to be highlytoxic
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a subst ...
in animal model
An animal model (short for animal disease model) is a living, non-human, often genetic-engineered animal used during the research and investigation of human disease, for the purpose of better understanding the disease process without the risk of ha ...
s ( of 2500 mg/kg for rat
Rats are various medium-sized, long-tailed rodents. Species of rats are found throughout the order Rodentia, but stereotypical rats are found in the genus ''Rattus''. Other rat genera include '' Neotoma'' (pack rats), '' Bandicota'' (bandicoo ...
s). However, recent observations of acute kidney injury following exposure to hair-straightening products indicate that it is toxic. After transcutaneous absorption, glyoxylic acid contained in hair-strengthening creams causes calcium oxalate
Calcium oxalate (in archaic terminology, oxalate of lime) is a calcium salt of oxalic acid with the chemical formula or . It forms hydrates , where ''n'' varies from 1 to 3. Anhydrous and all hydrated forms are colorless or white. The monohydr ...
nephropathy
Kidney disease, or renal disease, technically referred to as nephropathy, is damage to or disease of a kidney
In humans, the kidneys are two reddish-brown bean-shaped blood-filtering organ (anatomy), organs that are a multilobar, multipap ...
. In contrast to glycolic acid
Glycolic acid (or hydroxyacetic acid; chemical formula ) is a colorless, odorless and hygroscopic crystal, crystalline solid, highly solubility, soluble in water. It is used in various skin care, skin-care products. Glycolic acid is widespread in ...
, glyoxylic acid can dramatically increase urine oxalate
Oxalate (systematic IUPAC name: ethanedioate) is an anion with the chemical formula . This dianion is colorless. It occurs naturally, including in some foods. It forms a variety of salts, for example sodium oxalate (), and several esters such as ...
excretion
Excretion is elimination of metabolic waste, which is an essential process in all organisms. In vertebrates, this is primarily carried out by the lungs, Kidney (vertebrates), kidneys, and skin. This is in contrast with secretion, where the substa ...
.
See also
* SemialdehydeReferences